当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spin-paired solvated electron couples in alkali–ammonia systems†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1039/c8cp05058a Michael Mauksch 1, 2, 3, 4, 5 , Svetlana B. Tsogoeva 1, 5, 6, 7, 8
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1039/c8cp05058a Michael Mauksch 1, 2, 3, 4, 5 , Svetlana B. Tsogoeva 1, 5, 6, 7, 8
Affiliation
|
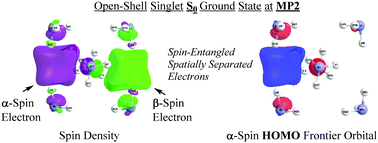
Alkali metals form deep blue solutions of solvated electrons in liquid ammonia. To explain the diamagnetism of more concentrated solutions, DFT and ab initio computations have been used to show that spin-paired couples of electrons can overcome Coulomb repulsion, occupying a cavity formed by solvent molecules, comparable in size to that of single solvated electrons. Sluggish hydrogen evolution from the solutions is rationalized by a high activation barrier for reaction of the electron pair with an ammonia molecule.
中文翻译:

碱-氨系统中自旋配对的溶剂化电子对†
碱金属在液氨中形成溶剂化电子的深蓝色溶液。为了解释更浓溶液的反磁性,已使用DFT和从头算的方法来显示,自旋配对的电子对可以克服库仑排斥,占据由溶剂分子形成的空腔,其大小可与单个溶剂化电子相媲美。从溶液中放出缓慢的氢是由于电子对与氨分子反应的高活化势垒所致。
更新日期:2018-10-17
中文翻译:

碱-氨系统中自旋配对的溶剂化电子对†
碱金属在液氨中形成溶剂化电子的深蓝色溶液。为了解释更浓溶液的反磁性,已使用DFT和从头算的方法来显示,自旋配对的电子对可以克服库仑排斥,占据由溶剂分子形成的空腔,其大小可与单个溶剂化电子相媲美。从溶液中放出缓慢的氢是由于电子对与氨分子反应的高活化势垒所致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号