当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A revisit of the interaction of gaseous ozone with aqueous iodide. Estimating the contributions of the surface and bulk reactions†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1039/c8cp04394a Carolina G. Moreno 1, 2, 3, 4 , Oscar Gálvez 4, 5, 6, 7, 8 , Vicente López-Arza Moreno 1, 2, 3, 4 , Eva María Espildora-García 1, 2, 3, 4 , María Teresa Baeza-Romero 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1039/c8cp04394a Carolina G. Moreno 1, 2, 3, 4 , Oscar Gálvez 4, 5, 6, 7, 8 , Vicente López-Arza Moreno 1, 2, 3, 4 , Eva María Espildora-García 1, 2, 3, 4 , María Teresa Baeza-Romero 1, 2, 3, 4
Affiliation
|
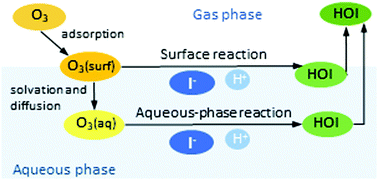
The main source of atmospheric iodine is the heterogeneous reaction of aqueous iodide (I−) with ozone (O3), which takes place in surface seawater and probably in sea-salt aerosols. However, there are seemingly contradictory conclusions about whether this heterogeneous reaction occurs in the bulk of the aqueous phase, via O3 dissolution, or at the aqueous surface, via O3 adsorption. In this work, the ozone uptake coefficient has been calculated as a function of the concentration of aqueous iodide ([I−]aq) and gaseous ozone near the aqueous surface ([O3]gs) by estimating parameters of the resistor model using results of previous studies. The calculated uptake coefficients suggest that the aqueous-phase reaction dominates at low I− concentrations (about <10−4 mol L−1), regardless of [O3]gs, and also at sufficiently high [O3]gs (about >80 ppm), regardless of [I−]aq. In contrast, the surface reaction dominates at high [I−]aq (about >10−4 mol L−1) as long as [O3]gs is low enough (about <80 ppm). This trend is able to reconcile previous studies of this reaction, and is a consequence of several factors, including the high surface excess of both reactants ozone and iodide. Given the typical O3 concentrations in the troposphere and the possible I− concentrations and O3 solubilities in sea-salt aerosols, the surface reaction may compete with the aqueous-phase reaction in accumulation-mode aerosols, unlike in surface seawater, where the aqueous-phase reaction probably prevails. The rate constant of the surface reaction has been estimated as (3–40) × 10−13 cm2 molecule−1 s−1.
中文翻译:

重温气态臭氧与碘水溶液的相互作用。估计表面反应和本体反应的贡献†
大气碘的主要来源是含水碘(I非均相反应- )与臭氧(O 3),其发生在表面海水并可能在海盐气溶胶。但是,关于这种非均相反应是发生在大部分水相中(通过O 3溶解)还是在水表面(通过O 3吸附)发生,似乎存在矛盾的结论。在这项工作中,吸收系数臭氧已被计算为含水碘的浓度的函数([I - ]水溶液)和气态臭氧附近的水性表面(〔O 3 ] GS),然后使用先前的研究结果估算电阻器模型的参数。摄取系数计算表明,水相反应占优势在低I -浓度(约<10 -4摩尔大号-1),不管〔O 3 ] GS,以及在足够高的〔O 3 ] GS(约>为80ppm),不管[I的- ]水溶液。相反,在高表面反应占主导地位[I - ]水溶液(约> 10 -4摩尔大号-1),只要〔O 3 ] GS足够低(约<80 ppm)。这种趋势能够调和先前对该反应的研究结果,并且是多种因素共同作用的结果,其中包括反应物臭氧和碘化物的表面高度过量。给定典型Ó 3倍在对流层的浓度和可能的I -浓度和O 3在海盐气溶胶溶解度,表面反应可以在表面海水,其中含水在积累型气溶胶的水相反应竞争,不像相反应可能占主导。表面反应的速率常数估计为(3-40)×10 -13 cm 2分子-1 s -1。
更新日期:2018-10-17
中文翻译:

重温气态臭氧与碘水溶液的相互作用。估计表面反应和本体反应的贡献†
大气碘的主要来源是含水碘(I非均相反应- )与臭氧(O 3),其发生在表面海水并可能在海盐气溶胶。但是,关于这种非均相反应是发生在大部分水相中(通过O 3溶解)还是在水表面(通过O 3吸附)发生,似乎存在矛盾的结论。在这项工作中,吸收系数臭氧已被计算为含水碘的浓度的函数([I - ]水溶液)和气态臭氧附近的水性表面(〔O 3 ] GS),然后使用先前的研究结果估算电阻器模型的参数。摄取系数计算表明,水相反应占优势在低I -浓度(约<10 -4摩尔大号-1),不管〔O 3 ] GS,以及在足够高的〔O 3 ] GS(约>为80ppm),不管[I的- ]水溶液。相反,在高表面反应占主导地位[I - ]水溶液(约> 10 -4摩尔大号-1),只要〔O 3 ] GS足够低(约<80 ppm)。这种趋势能够调和先前对该反应的研究结果,并且是多种因素共同作用的结果,其中包括反应物臭氧和碘化物的表面高度过量。给定典型Ó 3倍在对流层的浓度和可能的I -浓度和O 3在海盐气溶胶溶解度,表面反应可以在表面海水,其中含水在积累型气溶胶的水相反应竞争,不像相反应可能占主导。表面反应的速率常数估计为(3-40)×10 -13 cm 2分子-1 s -1。


























 京公网安备 11010802027423号
京公网安备 11010802027423号