当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Encapsulation versus Self-Aggregation toward Highly Selective Artificial K+ Channels
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1021/acs.accounts.8b00311 Mihail Barboiu 1, 2
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1021/acs.accounts.8b00311 Mihail Barboiu 1, 2
Affiliation
|
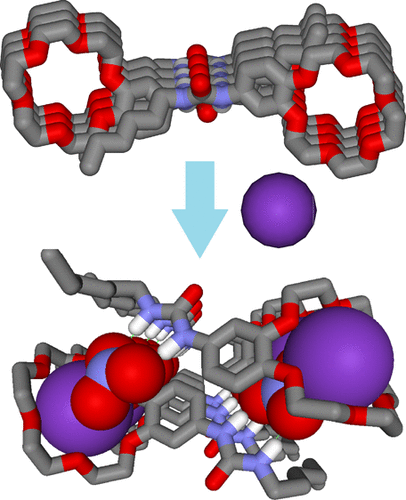
Natural ion-channel proteins allow ion transport across cell membranes at rates very close to those for ionic diffusion in water. Among them, natural KcsA K+ channels present high transport rates and total selectivity for K+ cations, rejecting all other cations. Most of the reported artificial ion channels cannot reach this type of activity because of their low selectivity. Several synthetic channels have been designed to mimic the natural KcSA channels, but those presenting an important K+/Na+ selectivity are limited. High-selectivity issues are determinant for the performance of natural protein channels, but they have been not considered as determinant in controlling the transport activity of the artificial ion channels. This Account discusses the last developments of artificial supramolecular carriers or channels that selectively transport K+ cations against other cations. Mimicking the complex structures of protein channels is an important research area. These studies are related to such adaptive biomimetic systems that can self-select their functions, with a specific emphasis on artificial superstructures enabling K+ transport like in the natural ones. Alternatively, it is more than interesting to synthetically construct only the active key structures of protein filters or gates that give the chemical selectivity or lead us to describe their dynamic role in the ion pumping and translocation along the channel. Several self-assembled macrocyclic channels are presented here. The macrocyclic binding sites may selectively encapsulate the K+ cations or form aggregated H-bonded central pores of self-assembled macrocycles that coordinate the K+ cations as hydrating water molecules in aqueous solution, compensating for the energetic cost of cation dehydration. These macrocyclic channels are responsive in the presence of K+ cations, even when a large excess of Na+ is present. From the mechanistic point of view, these systems express a synergistic dynamic feature: addition of K+ cations drives the selection and emergence of specific ion channels that selectively conduct the K+ cations that promoted the formation of channel superstructures in the first place. These highly permeable and K+-selective artificial channels may be considered as simple primitive biomimetic alternatives of natural KcsA channels that may find interesting applications in chemical separations, selective sensing, and biomedical materials.
中文翻译:

封装与自聚集对高选择性人工K +通道的影响
天然的离子通道蛋白可以使离子跨细胞膜转运,其速度非常接近离子在水中的扩散速度。其中,天然的KcsA K +通道呈现出高的迁移率和对K +阳离子的总选择性,拒绝了所有其他阳离子。大多数报道的人工离子通道由于其低选择性而无法达到这种活性。设计了几种合成通道来模仿天然的KcSA通道,但是那些通道表现出重要的K + / Na +选择性是有限的。高选择性问题是决定天然蛋白质通道性能的决定因素,但尚未考虑它们在控制人造离子通道的转运活性中起决定作用。该论文讨论了人工超分子载体或通道的最新发展,该载体或通道选择性地将K +阳离子转运到其他阳离子上。模仿蛋白质通道的复杂结构是重要的研究领域。这些研究涉及到这种自适应仿生系统,可以自行选择自己的功能,具有特别强调人工上层建筑使ķ +运输像在自然的。备选地,仅合成合成具有化学选择性或引导我们描述其在离子泵送和沿通道转运中的动态作用的蛋白质过滤器或门的活性关键结构,将是非常有意思的。这里介绍了几个自组装的大循环通道。大环结合位点可以选择性地包裹K +阳离子或形成自组装大环的聚集的H键合中心孔,这些中心孔在水溶液中水合水分子时协调K +阳离子,从而补偿了阳离子脱水的高能成本。这些大环通道在存在K +阳离子的情况下仍具有响应性,即使Na +大量过量存在。从机理的角度来看,这些系统表现出协同的动态特性:K +阳离子的添加驱动特定离子通道的选择和出现,这些离子通道选择性地传导K +阳离子,从而首先促进了通道超结构的形成。这些高渗透性且具有K +选择性的人工通道可被视为天然KcsA通道的简单原始仿生替代品,可在化学分离,选择性传感和生物医学材料中找到有趣的应用。
更新日期:2018-10-17
中文翻译:

封装与自聚集对高选择性人工K +通道的影响
天然的离子通道蛋白可以使离子跨细胞膜转运,其速度非常接近离子在水中的扩散速度。其中,天然的KcsA K +通道呈现出高的迁移率和对K +阳离子的总选择性,拒绝了所有其他阳离子。大多数报道的人工离子通道由于其低选择性而无法达到这种活性。设计了几种合成通道来模仿天然的KcSA通道,但是那些通道表现出重要的K + / Na +选择性是有限的。高选择性问题是决定天然蛋白质通道性能的决定因素,但尚未考虑它们在控制人造离子通道的转运活性中起决定作用。该论文讨论了人工超分子载体或通道的最新发展,该载体或通道选择性地将K +阳离子转运到其他阳离子上。模仿蛋白质通道的复杂结构是重要的研究领域。这些研究涉及到这种自适应仿生系统,可以自行选择自己的功能,具有特别强调人工上层建筑使ķ +运输像在自然的。备选地,仅合成合成具有化学选择性或引导我们描述其在离子泵送和沿通道转运中的动态作用的蛋白质过滤器或门的活性关键结构,将是非常有意思的。这里介绍了几个自组装的大循环通道。大环结合位点可以选择性地包裹K +阳离子或形成自组装大环的聚集的H键合中心孔,这些中心孔在水溶液中水合水分子时协调K +阳离子,从而补偿了阳离子脱水的高能成本。这些大环通道在存在K +阳离子的情况下仍具有响应性,即使Na +大量过量存在。从机理的角度来看,这些系统表现出协同的动态特性:K +阳离子的添加驱动特定离子通道的选择和出现,这些离子通道选择性地传导K +阳离子,从而首先促进了通道超结构的形成。这些高渗透性且具有K +选择性的人工通道可被视为天然KcsA通道的简单原始仿生替代品,可在化学分离,选择性传感和生物医学材料中找到有趣的应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号