当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
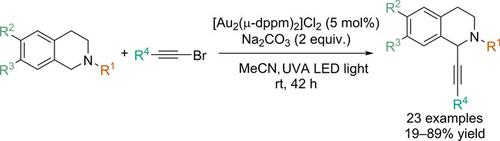
Gold Catalyzed Photoredox C1‐Alkynylation of N‐Alkyl‐1,2,3,4‐tetrahydroisoquinolines by 1‐Bromoalkynes with UVA LED Light
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-11-16 , DOI: 10.1002/adsc.201801289 Yichao Zhao 1 , Jianwen Jin 1 , Philip Wai Hong Chan 1, 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-11-16 , DOI: 10.1002/adsc.201801289 Yichao Zhao 1 , Jianwen Jin 1 , Philip Wai Hong Chan 1, 2
Affiliation
|
A synthetic method that combines [Au2(μ‐dppm)2]Cl2 (dppm=bis(diphenylphosphanyl)methane) and UVA LED (LED=light emitting diode) light (365 nm) to catalyze the regioselective C1‐alkynylation of N‐alkyl‐1,2,3,4‐tetrahydroisoquinolines (THIQs) with alkynyl bromides is described. The reaction mechanism was delineated to involve a reductive quench pathway to generate the two posited radical species of the nitrogen‐containing heterocycle and organic halide. In contrast, radical formation via an oxidative quench pathway was suggested to be operative in analogous control experiments with a 1‐iodoalkyne. The usefulness of this carbon‐carbon bond forming strategy was also exemplified by its application to the formal synthesis of the opioid analgesic drug methopholine and synthesis of a protoberberine alkaloid derivative.
中文翻译:

1-溴代炔烃与UVA LED灯金催化N-烷基-1,2,3,4-四氢异喹啉催化的光氧化还原C1-烷基化
一种合成方法,将[Au 2(μ-dppm)2 ] Cl 2(dppm =双(二苯基膦基)甲烷)和UVA LED(LED =发光二极管)光(365 nm)结合起来,催化N的区域选择性C1-炔基化描述了烷基,1,2,3,4,4-四氢异喹啉(THIQs)与炔基溴化物的关系。划定了反应机理,涉及一个还原性淬灭途径,以生成含氮杂环和有机卤化物的两个正自由基基团。相反,自由基通过有人建议在类似1-碘炔的对照实验中使用氧化猝灭途径。这种碳-碳键形成策略的有用性还通过将其应用于阿片类镇痛药甲氧磷的形式合成和原小ber碱生物碱衍生物的合成而得到了证明。
更新日期:2018-11-16
中文翻译:

1-溴代炔烃与UVA LED灯金催化N-烷基-1,2,3,4-四氢异喹啉催化的光氧化还原C1-烷基化
一种合成方法,将[Au 2(μ-dppm)2 ] Cl 2(dppm =双(二苯基膦基)甲烷)和UVA LED(LED =发光二极管)光(365 nm)结合起来,催化N的区域选择性C1-炔基化描述了烷基,1,2,3,4,4-四氢异喹啉(THIQs)与炔基溴化物的关系。划定了反应机理,涉及一个还原性淬灭途径,以生成含氮杂环和有机卤化物的两个正自由基基团。相反,自由基通过有人建议在类似1-碘炔的对照实验中使用氧化猝灭途径。这种碳-碳键形成策略的有用性还通过将其应用于阿片类镇痛药甲氧磷的形式合成和原小ber碱生物碱衍生物的合成而得到了证明。


























 京公网安备 11010802027423号
京公网安备 11010802027423号