当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Constructing the Phase Diagram of a Single-Component System Using Fundamental Principles of Thermodynamics and Statistical Mechanics: A Spreadsheet-Based Learning Experience for Students
Journal of Chemical Education ( IF 3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1021/acs.jchemed.8b00560 Arthur M. Halpern 1 , Charles J. Marzzacco 2
Journal of Chemical Education ( IF 3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1021/acs.jchemed.8b00560 Arthur M. Halpern 1 , Charles J. Marzzacco 2
Affiliation
|
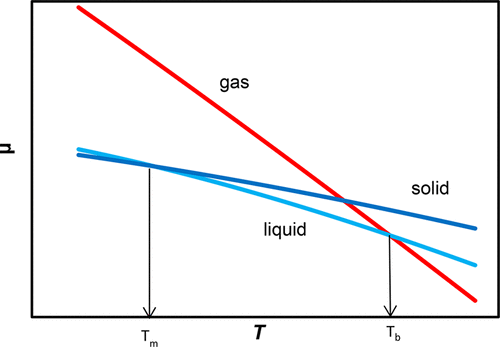
A spreadsheet project that enables students to construct chemical potential vs temperature plots, μ(T), of liquid and gaseous mercury and all three phases of diiodine and ammonia is presented. Statistical thermodynamics is used to determine the entropies of the substances in the gas phase. For the solid and liquid phases, students start with published heat capacities, third law entropies, and other thermodynamic quantities to obtain entropies over the range of temperatures needed. By using thermodynamic cycles, they calculate the chemical potentials of the metastable phases at standard conditions. With these values as starting points, students use the Gibbs equation and stepwise integration to obtain the chemical potentials over a range of temperatures. Using this data, they calculate the melting and boiling points and transition enthalpies and entropies from the intersection points of the two phases. The calculated thermochemical quantities are in excellent agreement with literature values.
中文翻译:

使用热力学和统计力学的基本原理构建单组分系统的相图:基于电子表格的学生学习经验
一个电子表格项目,使学生能够构建化学势与温度的关系图,μ(T),介绍了液态和气态汞以及二碘和氨的所有三个相。统计热力学用于确定气相中物质的熵。对于固相和液相,学生将从已公开的热容量,第三定律熵和其他热力学量入手,以获取所需温度范围内的熵。通过使用热力学循环,他们可以计算标准条件下亚稳相的化学势。以这些值为起点,学生可以使用Gibbs方程和逐步积分来获得在一定温度范围内的化学势。他们使用这些数据,根据两相交点计算熔点和沸点以及跃迁焓和熵。
更新日期:2018-10-17
中文翻译:

使用热力学和统计力学的基本原理构建单组分系统的相图:基于电子表格的学生学习经验
一个电子表格项目,使学生能够构建化学势与温度的关系图,μ(T),介绍了液态和气态汞以及二碘和氨的所有三个相。统计热力学用于确定气相中物质的熵。对于固相和液相,学生将从已公开的热容量,第三定律熵和其他热力学量入手,以获取所需温度范围内的熵。通过使用热力学循环,他们可以计算标准条件下亚稳相的化学势。以这些值为起点,学生可以使用Gibbs方程和逐步积分来获得在一定温度范围内的化学势。他们使用这些数据,根据两相交点计算熔点和沸点以及跃迁焓和熵。


























 京公网安备 11010802027423号
京公网安备 11010802027423号