当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective Hydrogenation of Biomass-Derived 2(5H)-Furanone to γ-Butyrolactone over Ni-Based Bimetallic Catalysts
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-10-15 00:00:00 , DOI: 10.1021/acssuschemeng.8b02212 Xiaodan Li 1, 2 , Weiming Wan 2 , Jingguang G. Chen 2, 3 , Tiefeng Wang 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-10-15 00:00:00 , DOI: 10.1021/acssuschemeng.8b02212 Xiaodan Li 1, 2 , Weiming Wan 2 , Jingguang G. Chen 2, 3 , Tiefeng Wang 1
Affiliation
|
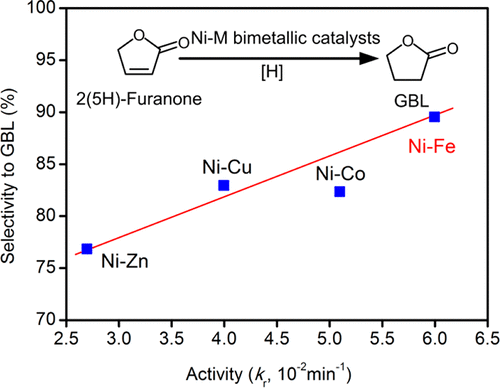
2(5H)-Furanone was selectively hydrogenated to γ-butyrolactone (GBL) over Ni-based bimetallic catalysts, Ni-M/SiO2 (M = Fe, Co, Cu, and Zn). Catalyst characterization showed that the metal dispersion and reducibility were changed after introducing a second metal, while the physical morphology was nearly unchanged. The most active and selective bimetallic catalyst was found to be Ni–Fe/SiO2. The reaction rate constant over the 4 wt %Ni–1 wt %Fe/SiO2 bimetallic catalyst was 0.06 min–1, doubling that over 5 wt %Ni/SiO2, and the selectivity to GBL increased slightly from 86.1 to 89.5%. The Fe/(Ni+Fe) weight ratio was changed to optimize the promoting effect of Fe. The Ni–Fe bimetallic effect was further investigated using temperature-programmed desorption experiments on single crystal model surfaces with different Fe/Ni(111) structures, revealing that the enhanced catalyst activity was mainly contributed by the formation of the Ni–Fe surface alloy.
中文翻译:

镍基双金属催化剂上生物质衍生的2(5H)-呋喃酮选择性加氢为γ-丁内酯
在Ni基双金属催化剂Ni-M / SiO 2(M = Fe,Co,Cu和Zn)上,将2(5H)-呋喃酮选择性加氢成γ-丁内酯(GBL )。催化剂表征表明,引入第二种金属后,金属的分散性和还原性发生了变化,而物理形态几乎没有变化。发现最具活性和选择性的双金属催化剂是Ni-Fe / SiO 2。在4 wt%Ni–1 wt%Fe / SiO 2双金属催化剂上的反应速率常数为0.06 min –1,是在5 wt%Ni / SiO 2上的反应速率常数的两倍,对GBL的选择性从86.1略微提高到89.5%。改变Fe /(Ni + Fe)的重量比以优化Fe的促进效果。使用温度程序解吸实验对具有不同Fe / Ni(111)结构的单晶模型表面进一步研究了Ni-Fe双金属效应,发现增强的催化剂活性主要是由Ni-Fe表面合金的形成引起的。
更新日期:2018-10-15
中文翻译:

镍基双金属催化剂上生物质衍生的2(5H)-呋喃酮选择性加氢为γ-丁内酯
在Ni基双金属催化剂Ni-M / SiO 2(M = Fe,Co,Cu和Zn)上,将2(5H)-呋喃酮选择性加氢成γ-丁内酯(GBL )。催化剂表征表明,引入第二种金属后,金属的分散性和还原性发生了变化,而物理形态几乎没有变化。发现最具活性和选择性的双金属催化剂是Ni-Fe / SiO 2。在4 wt%Ni–1 wt%Fe / SiO 2双金属催化剂上的反应速率常数为0.06 min –1,是在5 wt%Ni / SiO 2上的反应速率常数的两倍,对GBL的选择性从86.1略微提高到89.5%。改变Fe /(Ni + Fe)的重量比以优化Fe的促进效果。使用温度程序解吸实验对具有不同Fe / Ni(111)结构的单晶模型表面进一步研究了Ni-Fe双金属效应,发现增强的催化剂活性主要是由Ni-Fe表面合金的形成引起的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号