Biomaterials ( IF 14.0 ) Pub Date : 2018-10-15 , DOI: 10.1016/j.biomaterials.2018.10.016 Ruyi Zhou , Huamei Wang , Yufei Yang , Chenyang Zhang , Xinghua Dong , Jiangfeng Du , Liang Yan , Guangjin Zhang , Zhanjun Gu , Yuliang Zhao
|
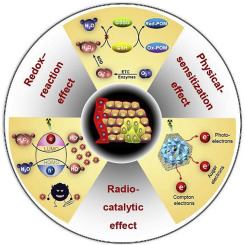
Radioresistance resulted from the intrinsic features of tumors often gives rise to unsatisfied therapeutic outcome. In particular, the tumor microenvironment (TME) with abundant antioxidants, elevated hydrogen peroxide (H2O2) and hypoxia has been believed as a tremendous obstacle for radiotherapy. Therefore, developing an effective radiosensitizer in response to both X-ray and the TME is highly imperative but remains a challenge so far. Here, we for the first time explore bismuth heteropolytungstate (BiP5W30) nanoclusters as radiosensitizers for the TME-manipulated enhancement of radiotherapy. On the one hand, BiP5W30 nanoclusters can increase radiation dose deposition within tumors by high-Z elements like Bi and W. On the other hand, in virtue of the unique electron structure and multi-electron property, they have the capability of depleting glutathione (GSH) via redox reaction and catalyzing the decomposition of H2O2 to HO to enhance ROS generation upon X-ray radiation. Moreover, reduced graphene oxide (rGO) coupled with BiP5W30 can further improve radiocatalytic activity through promoting electron-hole separation. Simultaneously, due to the considerable near-infrared absorption of rGO, photothermal therapy can overcome the tumor hypoxia microenvironment and thus synergize with radiotherapy. In addition to providing a promising radiosensitizer, this finding is expected to extend the application of polyoxometalates used in the biomedical field.
中文翻译:

基于异钨酸铋的肿瘤微环境操纵的放射催化增敏剂
由肿瘤的内在特征引起的抗辐射性常常引起不满意的治疗结果。特别地,具有丰富的抗氧化剂,升高的过氧化氢(H 2 O 2)和低氧的肿瘤微环境(TME)被认为是放疗的巨大障碍。因此,迫切需要开发一种对X射线和TME都有效的放射增敏剂,但迄今为止仍然是一个挑战。在这里,我们第一次探索铋异多钨酸铋(BiP 5 W 30)纳米簇作为放射增敏剂,以TME操纵增强放射治疗。一方面,BiP 5 W 30纳米团簇可以通过Bi和W等高Z元素来增加肿瘤内的辐射剂量沉积。另一方面,由于独特的电子结构和多电子性质,它们具有通过氧化还原反应消耗谷胱甘肽(GSH)的能力。催化H 2 O 2分解为HO,以增强X射线辐射时ROS的生成。此外,还原的氧化石墨烯(rGO)与BiP 5 W 30耦合可以通过促进电子-空穴分离来进一步提高放射催化活性。同时,由于rGO的近红外吸收,光热疗法可以克服肿瘤缺氧的微环境,从而与放射疗法协同作用。除了提供有希望的放射增敏剂外,预期该发现还将扩展生物医学领域中使用的多金属氧酸盐的应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号