当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
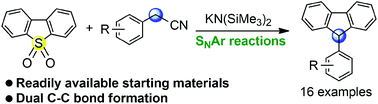
KHMDS mediated synthesis of 9-arylfluorenes from dibenzothiophene dioxides and arylacetonitriles by tandem SNAr-decyanation-based arylation
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-10-15 , DOI: 10.1039/c8ob02355g Saketh Mylavarapu 1, 2, 3, 4, 5 , Mamta Yadav 1, 2, 3, 4, 5 , M. Bhanuchandra 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-10-15 , DOI: 10.1039/c8ob02355g Saketh Mylavarapu 1, 2, 3, 4, 5 , Mamta Yadav 1, 2, 3, 4, 5 , M. Bhanuchandra 1, 2, 3, 4, 5
Affiliation
|
A straightforward KHMDS mediated synthetic route to 9-arylfluorenes from readily available starting materials has been developed. This reaction involves SNAr reactions of dioxide with arylacetonitriles, followed by decyanation reaction. The proposed transformation can also be used to furnish a densely arylated indene.
中文翻译:

KHMDS介导串联S N Ar脱氰基芳基化反应由二苯并噻吩二氧化物和芳基乙腈合成9-芳基芴
已经开发了一种简单的由KHMDS介导的从容易获得的起始原料合成9-芳基芴的合成路线。该反应包括小号Ñ二氧化arylacetonitriles的氩反应,随后脱氰反应。所提出的转化也可以用于提供致密的芳基化的茚。
更新日期:2018-11-02
中文翻译:

KHMDS介导串联S N Ar脱氰基芳基化反应由二苯并噻吩二氧化物和芳基乙腈合成9-芳基芴
已经开发了一种简单的由KHMDS介导的从容易获得的起始原料合成9-芳基芴的合成路线。该反应包括小号Ñ二氧化arylacetonitriles的氩反应,随后脱氰反应。所提出的转化也可以用于提供致密的芳基化的茚。



























 京公网安备 11010802027423号
京公网安备 11010802027423号