当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insights into Substrate Selectivity and Activity of Bacterial Polyphosphate Kinases
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-10-12 00:00:00 , DOI: 10.1021/acscatal.8b03151 Boguslaw P. Nocek 1 , Anna N. Khusnutdinova 2 , Milosz Ruszkowski 3 , Robert Flick 2 , Malgorzata Burda 4 , Khorcheska Batyrova 2 , Greg Brown 2 , Artur Mucha 4 , Andrzej Joachimiak 1 , Łukasz Berlicki 4 , Alexander F. Yakunin 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-10-12 00:00:00 , DOI: 10.1021/acscatal.8b03151 Boguslaw P. Nocek 1 , Anna N. Khusnutdinova 2 , Milosz Ruszkowski 3 , Robert Flick 2 , Malgorzata Burda 4 , Khorcheska Batyrova 2 , Greg Brown 2 , Artur Mucha 4 , Andrzej Joachimiak 1 , Łukasz Berlicki 4 , Alexander F. Yakunin 2
Affiliation
|
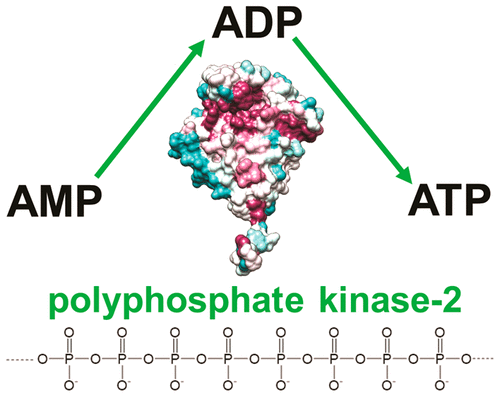
Polyphosphate (polyP) kinases are widely conserved enzymes with importance in basic bacterial metabolism and virulence in many pathogens. However, the molecular mechanisms of their substrate specificity and catalysis remain unknown. Here, we present the results of comprehensive biochemical and structural studies of three polyP kinases from different bacteria, which belong to different clusters of the PPK2 class III family. Purified PPK2 proteins catalyzed polyP-dependent phosphorylation of AMP, ADP, GMP, and GDP to corresponding nucleoside diphosphates and triphosphates. Crystal structures of these proteins in complex with substrates, products, Mg2+, and inhibitors revealed the binding sites for the nucleotide and polyP substrates overlapping at the Walker A and B loops. The Walker A loop is involved in the binding of polyP and the Mg2+ ion, whereas the Walker B loop coordinates the nucleotide phosphate groups. Structure-based site-directed mutagenesis of CHU0107 from Cytophaga hutchinsonii demonstrated the critical role of several conserved residues from the PPK2 core and lid domains, which are involved in the coordination of both substrates and two Mg2+ ions. In addition, a two-times higher activity was observed following deletion of the C-terminal tail in the CHU0107 mutant protein L285Stop. Crystal structures of PPK2 in complex with three aryl phosphonate inhibitors indicated the presence of at least two binding pockets for inhibitors located close to the Walker A loop and the catalytic residues Lys81 and Arg208. Our findings provide a molecular framework for understanding the molecular mechanisms of PPK2 kinases and have implications for future drug design and biocatalytic applications.
中文翻译:

细菌多磷酸激酶底物选择性和活性的结构见解。
聚磷酸(polyP)激酶是广泛保存的酶,在许多病原体的基本细菌代谢和毒力中具有重要意义。然而,其底物特异性和催化作用的分子机理仍然未知。在这里,我们介绍了来自不同细菌的三种polyP激酶的全面生化和结构研究的结果,这些细菌属于PPK2 III类家族的不同簇。纯化的PPK2蛋白催化AMP,ADP,GMP和GDP的polyP依赖磷酸化为相应的核苷二磷酸和三磷酸。这些蛋白质的晶体结构与底物,产物,Mg 2+形成复合物和抑制剂揭示了在Walker A和B环处重叠的核苷酸和polyP底物的结合位点。Walker A环参与了polyP和Mg 2+离子的结合,而Walker B环则协调了核苷酸磷酸基团。来自Cytophaga hutchinsonii的CHU0107的基于结构的定点诱变表明,来自PPK2核心和盖结构域的几个保守残基的关键作用,与两个底物和两个Mg 2+的配位有关离子。此外,在CHU0107突变蛋白L285Stop中删除了C末端尾部后,观察到了两倍高的活性。与三种芳基膦酸酯抑制剂复合的PPK2的晶体结构表明,存在至少两个位于沃克A环附近的抑制剂以及催化残基Lys81和Arg208的结合袋。我们的发现为理解PPK2激酶的分子机制提供了分子框架,并且对未来的药物设计和生物催化应用具有影响。
更新日期:2018-10-12
中文翻译:

细菌多磷酸激酶底物选择性和活性的结构见解。
聚磷酸(polyP)激酶是广泛保存的酶,在许多病原体的基本细菌代谢和毒力中具有重要意义。然而,其底物特异性和催化作用的分子机理仍然未知。在这里,我们介绍了来自不同细菌的三种polyP激酶的全面生化和结构研究的结果,这些细菌属于PPK2 III类家族的不同簇。纯化的PPK2蛋白催化AMP,ADP,GMP和GDP的polyP依赖磷酸化为相应的核苷二磷酸和三磷酸。这些蛋白质的晶体结构与底物,产物,Mg 2+形成复合物和抑制剂揭示了在Walker A和B环处重叠的核苷酸和polyP底物的结合位点。Walker A环参与了polyP和Mg 2+离子的结合,而Walker B环则协调了核苷酸磷酸基团。来自Cytophaga hutchinsonii的CHU0107的基于结构的定点诱变表明,来自PPK2核心和盖结构域的几个保守残基的关键作用,与两个底物和两个Mg 2+的配位有关离子。此外,在CHU0107突变蛋白L285Stop中删除了C末端尾部后,观察到了两倍高的活性。与三种芳基膦酸酯抑制剂复合的PPK2的晶体结构表明,存在至少两个位于沃克A环附近的抑制剂以及催化残基Lys81和Arg208的结合袋。我们的发现为理解PPK2激酶的分子机制提供了分子框架,并且对未来的药物设计和生物催化应用具有影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号