当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mass spectrometric studies of Cu(I)-binding to the N-terminal domains of B. subtilis CopA and influence of bacillithiol.
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2018-10-13 , DOI: 10.1016/j.jinorgbio.2018.10.004 Kristine L Kay 1 , Chris J Hamilton 2 , Nick E Le Brun 1
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2018-10-13 , DOI: 10.1016/j.jinorgbio.2018.10.004 Kristine L Kay 1 , Chris J Hamilton 2 , Nick E Le Brun 1
Affiliation
|
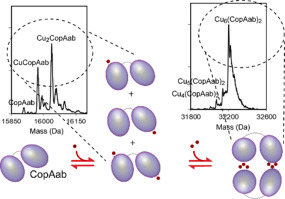
CopA is a Cu(I)-exporting transmembrane P1B-type ATPase from Bacillus subtilis. It contains two N-terminal cytoplasmic domains, CopAab, which bind Cu(I) with high affinity and to form higher-order complexes with multiple Cu(I) ions. To determine the precise nature of these species, electrospray ionisation mass spectrometry (ESI-MS) under non-denaturing conditions was employed. Up to 1 Cu per CopAab resulted in Cu coordination to one or both CopAab domains. At >1 Cu/CopAab, two distinct dimeric charge state envelopes were observed, corresponding to distinct conformations, each with Cu6(CopAab)2 as its major form. The influence of the physiologically relevant low molecular weight thiol bacillithiol (BSH) on Cu(I)-binding to CopAab was assessed. Dimeric CopAab persisted in the presence of BSH, with previously undetected Cu7(CopAab)2 and Cu6(CopAab)2(BSH) forms apparent.
中文翻译:

质谱研究Cu(I)结合到枯草芽孢杆菌CopA的N末端域和杆菌硫醇的影响。
CopA是来自枯草芽孢杆菌的Cu(I)出口跨膜P1B型ATPase。它包含两个N末端胞质结构域CopAab,它们以高亲和力结合Cu(I),并与多个Cu(I)离子形成更高阶的复合物。为了确定这些物质的精确性质,采用了非变性条件下的电喷雾电离质谱(ESI-MS)。每个CopAab多达1个Cu,导致Cu配位至一个或两个CopAab域。在> 1 Cu / CopAab处,观察到两个不同的二聚体电荷状态包膜,对应于不同的构象,每个都以Cu6(CopAab)2为主要形式。评估了生理相关的低分子量硫醇杆菌硫醇(BSH)对与CopAab结合的Cu(I)的影响。二聚体CopAab在BSH存在下持续存在,
更新日期:2018-10-13
中文翻译:

质谱研究Cu(I)结合到枯草芽孢杆菌CopA的N末端域和杆菌硫醇的影响。
CopA是来自枯草芽孢杆菌的Cu(I)出口跨膜P1B型ATPase。它包含两个N末端胞质结构域CopAab,它们以高亲和力结合Cu(I),并与多个Cu(I)离子形成更高阶的复合物。为了确定这些物质的精确性质,采用了非变性条件下的电喷雾电离质谱(ESI-MS)。每个CopAab多达1个Cu,导致Cu配位至一个或两个CopAab域。在> 1 Cu / CopAab处,观察到两个不同的二聚体电荷状态包膜,对应于不同的构象,每个都以Cu6(CopAab)2为主要形式。评估了生理相关的低分子量硫醇杆菌硫醇(BSH)对与CopAab结合的Cu(I)的影响。二聚体CopAab在BSH存在下持续存在,



























 京公网安备 11010802027423号
京公网安备 11010802027423号