Chem ( IF 23.5 ) Pub Date : 2018-10-11 , DOI: 10.1016/j.chempr.2018.09.004 Jiaxin Zheng , Guoyu Tan , Peng Shan , Tongchao Liu , Jiangtao Hu , Yancong Feng , Luyi Yang , Mingjian Zhang , Zonghai Chen , Yuan Lin , Jun Lu , Joerg C. Neuefeind , Yang Ren , Khalil Amine , Lin-Wang Wang , Kang Xu , Feng Pan
|
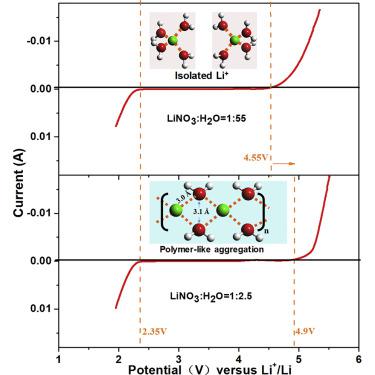
Aqueous electrolytes come with an intrinsic narrow electrochemical stability window (1.23 V). Expanding this window represents significant benefits in both fundamental science and practical battery applications. Recent breakthroughs made via super-concentration have resulted in >3.0 V windows, but fundamental understanding of the related mechanism is still absent. In the present work, we examined the widened window (2.55 V) of a super-concentrated (unsaturated) aqueous solution of LiNO3 through both theoretical and spectral analyses and discovered that a local structure of intimate Li+-water interaction arises at super-concentration, generating (Li+(H2O)2)n polymer-like chains to replace the ubiquitous hydrogen bonding between water molecules. Such structure is mainly responsible for the expanded electrochemical stability window. Further theoretical and experimental analyses quantitatively differentiate the contributions to this window, identifying the kinetic factor (desolvation) as the main contributor. Such molecular-level and quantitative understanding will further assist in tailor designing more effective approaches to stabilizing water electrochemically.
中文翻译:

了解扩大水电解质稳定性窗口的热力学和动力学贡献
水性电解质具有固有的狭窄电化学稳定性窗口(1.23 V)。扩大此窗口代表了基础科学和实际电池应用的重大利益。通过超浓缩取得的最新突破导致了> 3.0 V的窗口,但是仍然缺乏对相关机制的基本了解。在目前的工作中,我们通过理论和光谱分析研究了LiNO 3超浓缩(不饱和)水溶液的加宽窗口(2.55 V),并发现在Li- 3上存在紧密的Li +-水相互作用的局部结构。浓度,生成(Li +(H 2 O)2)n类聚合物链取代水分子之间普遍存在的氢键。这种结构主要负责扩大电化学稳定性窗口。进一步的理论和实验分析从数量上区分了对该窗口的贡献,确定了动力学因素(去溶剂化)为主要贡献者。这种分子水平和定量的理解将进一步有助于定制设计更有效的方法来电化学稳定水。



























 京公网安备 11010802027423号
京公网安备 11010802027423号