当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trapping iron(III)-oxo species at the boundary of the ‘Oxo Wall’: insights into the nature of the Fe(III)–O bond
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-10 , DOI: 10.1021/jacs.8b08950 Erik Andris 1 , Rafael Navrátil 1 , Juraj Jašík 1 , Mayank Puri 2 , Miquel Costas 3 , Lawrence Que 2 , Jana Roithová 1, 4
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-10 , DOI: 10.1021/jacs.8b08950 Erik Andris 1 , Rafael Navrátil 1 , Juraj Jašík 1 , Mayank Puri 2 , Miquel Costas 3 , Lawrence Que 2 , Jana Roithová 1, 4
Affiliation
|
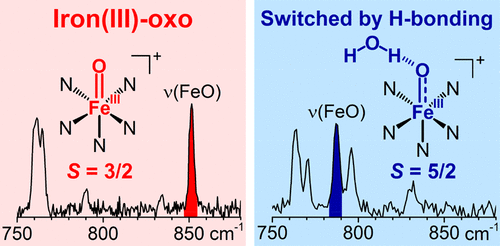
Terminal non-heme iron(IV)-oxo compounds are among the most powerful and best studied oxidants of strong C-H bonds. In contrast to the increasing number of such complexes (>80 thus far), corresponding one-electron-reduced derivatives are much rarer and presumably less stable, and only two iron(III)-oxo complexes have been characterized to date, both of which are stabilized by hydrogen-bonding interactions. Herein we have employed gas-phase techniques to generate and identify a series of terminal iron(III)-oxo complexes, all without built-in hydrogen bonding. Some of these complexes exhibit ∼70 cm-1 decrease in ν(Fe-O) frequencies expected for a half-order decrease in bond order upon one-electron reduction to an S = 5/2 center, while others have ν(Fe-O) frequencies essentially unchanged from those of their parent iron(IV)-oxo complexes. The latter result suggests that the added electron does not occupy a d orbital with Fe═O antibonding character, requiring an S = 3/2 spin assignment for the nascent FeIII-O- species. In the latter cases, water is found to hydrogen bond to the FeIII-O- unit, resulting in a change from quartet to sextet spin state. Reactivity studies also demonstrate the extraordinary basicity of these iron(III)-oxo complexes. Our observations show that metal-oxo species at the boundary of the "Oxo Wall" are accessible, and the data provide a lead to detect iron(III)-oxo intermediates in biological and biomimetic reactions.
中文翻译:

在“氧壁”边界捕获铁(III)-氧物种:深入了解 Fe(III)-O 键的性质
末端非血红素铁 (IV)-氧代化合物是最强大且研究最深入的强 CH 键氧化剂之一。与越来越多的此类配合物(迄今已超过 80 种)相比,相应的单电子还原衍生物要少得多,而且可能不太稳定,迄今为止,只有两种铁(III)-氧代配合物被表征,这两种由氢键相互作用稳定。在这里,我们采用气相技术来生成和识别一系列末端铁 (III)-氧配合物,所有这些都没有内置的氢键。这些配合物中的一些表现出 ν(Fe-O) 频率降低约 70 cm-1,预期在单电子还原到 S = 5/2 中心时键序降低半级,而其他配合物则具有 ν(Fe- O) 频率与其母体铁 (IV)-氧代复合物的频率基本相同。后一个结果表明,添加的电子不占据具有 Fe=O 反键特性的 ad 轨道,因此新生的 FeIII-O- 物种需要 S = 3/2 自旋分配。在后一种情况下,发现水与 FeIII-O- 单元形成氢键,导致自旋状态从四重态变为六重态。反应性研究还证明了这些铁 (III)-氧代配合物的非凡碱性。我们的观察表明,“氧壁”边界处的金属氧物种是可以访问的,这些数据为检测生物和仿生反应中的铁 (III)-氧中间体提供了线索。发现水与 FeIII-O- 单元形成氢键,导致自旋状态从四重态变为六重态。反应性研究还证明了这些铁 (III)-氧代配合物的非凡碱性。我们的观察表明,“氧壁”边界处的金属氧物种是可以访问的,这些数据为检测生物和仿生反应中的铁 (III)-氧中间体提供了线索。发现水与 FeIII-O- 单元形成氢键,导致自旋状态从四重态变为六重态。反应性研究还证明了这些铁 (III)-氧代配合物的非凡碱性。我们的观察表明,“氧壁”边界处的金属氧物种是可以访问的,这些数据为检测生物和仿生反应中的铁 (III)-氧中间体提供了线索。
更新日期:2018-10-10
中文翻译:

在“氧壁”边界捕获铁(III)-氧物种:深入了解 Fe(III)-O 键的性质
末端非血红素铁 (IV)-氧代化合物是最强大且研究最深入的强 CH 键氧化剂之一。与越来越多的此类配合物(迄今已超过 80 种)相比,相应的单电子还原衍生物要少得多,而且可能不太稳定,迄今为止,只有两种铁(III)-氧代配合物被表征,这两种由氢键相互作用稳定。在这里,我们采用气相技术来生成和识别一系列末端铁 (III)-氧配合物,所有这些都没有内置的氢键。这些配合物中的一些表现出 ν(Fe-O) 频率降低约 70 cm-1,预期在单电子还原到 S = 5/2 中心时键序降低半级,而其他配合物则具有 ν(Fe- O) 频率与其母体铁 (IV)-氧代复合物的频率基本相同。后一个结果表明,添加的电子不占据具有 Fe=O 反键特性的 ad 轨道,因此新生的 FeIII-O- 物种需要 S = 3/2 自旋分配。在后一种情况下,发现水与 FeIII-O- 单元形成氢键,导致自旋状态从四重态变为六重态。反应性研究还证明了这些铁 (III)-氧代配合物的非凡碱性。我们的观察表明,“氧壁”边界处的金属氧物种是可以访问的,这些数据为检测生物和仿生反应中的铁 (III)-氧中间体提供了线索。发现水与 FeIII-O- 单元形成氢键,导致自旋状态从四重态变为六重态。反应性研究还证明了这些铁 (III)-氧代配合物的非凡碱性。我们的观察表明,“氧壁”边界处的金属氧物种是可以访问的,这些数据为检测生物和仿生反应中的铁 (III)-氧中间体提供了线索。发现水与 FeIII-O- 单元形成氢键,导致自旋状态从四重态变为六重态。反应性研究还证明了这些铁 (III)-氧代配合物的非凡碱性。我们的观察表明,“氧壁”边界处的金属氧物种是可以访问的,这些数据为检测生物和仿生反应中的铁 (III)-氧中间体提供了线索。



























 京公网安备 11010802027423号
京公网安备 11010802027423号