当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functional Group Composition of Secondary Organic Aerosol Formed from Ozonolysis of α-Pinene Under High VOC and Autoxidation Conditions
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00117 Megan S. Claflin 1, 2 , Jordan E. Krechmer 1, 2 , Weiwei Hu 1, 2 , Jose L. Jimenez 1, 2 , Paul J. Ziemann 1, 2
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00117 Megan S. Claflin 1, 2 , Jordan E. Krechmer 1, 2 , Weiwei Hu 1, 2 , Jose L. Jimenez 1, 2 , Paul J. Ziemann 1, 2
Affiliation
|
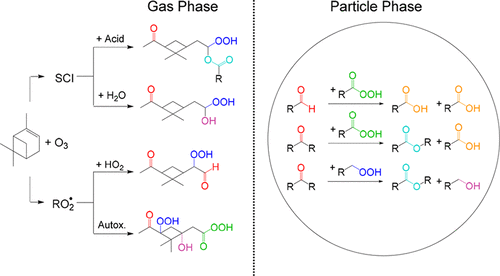
The formation of secondary organic aerosol (SOA) from α-pinene ozonolysis has been widely studied, with a recent focus on contributions from highly oxidized multifunctional compounds (HOMs) that have been observed in laboratory and field studies. Most of what is known about the chemical composition of SOA and HOMs, however, consists of molecular formulas and limited molecular structure identification based on mass spectrometric analysis. Here, we characterized the SOA formed from α-pinene ozonolysis using derivatization-spectrophotometric methods to quantify peroxide, carbonyl, carboxyl, ester, and hydroxyl groups. Experiments were conducted over a range of α-pinene concentrations and relative humidities, including regimes in which gas-phase HOMs were detected using NO3– chemical ionization mass spectrometry. Results for experiments conducted with high concentrations of α-pinene were also compared with predictions of a model that employed the Master Chemical Mechanism and included gas-particle and gas-wall partitioning. It appears that gas-phase monomer and dimer products formed through RO2• + RO2•, RO2• + HO2, RO2• isomerization, and stabilized Criegee intermediate + carboxylic acid or water reactions contributed to SOA formation, but that in particles the aldehyde and ketone groups in these compounds were often converted to carboxyl and ester groups through Baeyer–Villiger reactions with hydroperoxides and peroxycarboxylic acids. Evidence also indicates that hydrolysis of dimers containing diacyl peroxide groups contributed to the formation of carboxyl and ester groups, that hydroxyl groups were less abundant in SOA than expected (because of minor gas-phase alkoxy radical isomerization or conversion to an undetectable acetal oligomer), and that gas-to-particle partitioning of small carbonyl compounds may have contributed to SOA.
中文翻译:

高挥发性有机化合物和自氧化条件下由α-O烯的臭氧分解形成的次级有机气溶胶的官能团组成
已经广泛研究了由α-pine烯臭氧分解形成次要有机气溶胶(SOA),最近的重点是在实验室和现场研究中观察到的高氧化多功能化合物(HOM)的贡献。但是,关于SOA和HOM的化学组成的大多数已知信息都包括分子式和基于质谱分析的有限分子结构鉴定。在这里,我们表征了使用衍生化分光光度法从α-pine烯臭氧分解形成的SOA,以量化过氧化物,羰基,羧基,酯和羟基。实验在一定范围的α-pine烯浓度和相对湿度下进行,包括使用NO 3-检测气相HOM的方案。化学电离质谱。高浓度的α-pine烯进行的实验结果也与采用主化学机理并包括气体颗粒和气体壁分配的模型的预测结果进行了比较。看起来通过RO 2 • + RO 2 •,RO 2 • + HO 2,RO 2 •形成的气相单体和二聚体产物异构化和稳定的Criegee中间体+羧酸或水反应有助于SOA的形成,但是在颗粒中,这些化合物中的醛基和酮基通常通过与氢过氧化物和过氧羧酸的Baeyer-Villiger反应转化为羧基和酯基。证据还表明,含有二酰基过氧化物基团的二聚体的水解促进了羧基和酯基的形成,SOA中的羟基含量比预期的要少(由于较小的气相烷氧基自由基异构化或转化为不可检测的乙缩醛低聚物),而且小羰基化合物在气体与颗粒之间的分配可能促成了SOA。
更新日期:2018-10-10
中文翻译:

高挥发性有机化合物和自氧化条件下由α-O烯的臭氧分解形成的次级有机气溶胶的官能团组成
已经广泛研究了由α-pine烯臭氧分解形成次要有机气溶胶(SOA),最近的重点是在实验室和现场研究中观察到的高氧化多功能化合物(HOM)的贡献。但是,关于SOA和HOM的化学组成的大多数已知信息都包括分子式和基于质谱分析的有限分子结构鉴定。在这里,我们表征了使用衍生化分光光度法从α-pine烯臭氧分解形成的SOA,以量化过氧化物,羰基,羧基,酯和羟基。实验在一定范围的α-pine烯浓度和相对湿度下进行,包括使用NO 3-检测气相HOM的方案。化学电离质谱。高浓度的α-pine烯进行的实验结果也与采用主化学机理并包括气体颗粒和气体壁分配的模型的预测结果进行了比较。看起来通过RO 2 • + RO 2 •,RO 2 • + HO 2,RO 2 •形成的气相单体和二聚体产物异构化和稳定的Criegee中间体+羧酸或水反应有助于SOA的形成,但是在颗粒中,这些化合物中的醛基和酮基通常通过与氢过氧化物和过氧羧酸的Baeyer-Villiger反应转化为羧基和酯基。证据还表明,含有二酰基过氧化物基团的二聚体的水解促进了羧基和酯基的形成,SOA中的羟基含量比预期的要少(由于较小的气相烷氧基自由基异构化或转化为不可检测的乙缩醛低聚物),而且小羰基化合物在气体与颗粒之间的分配可能促成了SOA。



























 京公网安备 11010802027423号
京公网安备 11010802027423号