Journal of Environmental Management ( IF 8.7 ) Pub Date : 2018-10-11 , DOI: 10.1016/j.jenvman.2018.09.079 Huu Tap Van , Lan Huong Nguyen , Van Dang Nguyen , Xuan Hoan Nguyen , Thanh Hai Nguyen , Tien Vinh Nguyen , Saravanamuth Vigneswaran , Jörg Rinklebe , Hai Nguyen Tran
|
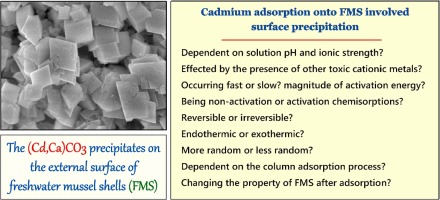
Calcium carbonate (CaCO3)-enriched biomaterial derived from freshwater mussel shells (FMS) was used as a non-porous biosorbent to explore the characteristics and mechanisms of cadmium adsorption in aqueous solution. The adsorption mechanism was proposed by comparing the FMS properties before and after adsorption alongside various adsorption studies. The FMS biosorbent was characterized using nitrogen adsorption/desorption isotherm, X-ray diffraction, scanning electron microscopy with energy dispersive spectroscopy, Fourier-transform infrared spectroscopy, and point of zero charge. The results of batch experiments indicated that FMS possessed an excellent affinity to Cd(II) ions within solutions pH higher than 4.0. An increase in ionic strength resulted in a significant decrease in the amount of Cd(II) adsorbed onto FMS. Kinetic study demonstrated that the adsorption process quickly reached equilibrium at approximately 60 min. The FMS biosorbent exhibited the Langmuir maximum adsorption capacity as follows: 18.2 mg/g at 10 °C < 26.0 mg/g at 30 °C < 28.6 mg/g at 50 °C. The Cd(II) adsorption process was irreversible, spontaneous (−ΔG°), endothermic (+ΔH°), and more random (+ΔS°). Selective order (mmol/g) of metal cations followed as Pb2+ > Cd2+ > Cu2+ > Cr3+ > Zn2+. For column experiments, the highest Thomas adsorption capacity (7.86 mg/g) was achieved at a flow rate (9 mL/min), initial Cd(II) concentration (10 mg/L), and bed height (5 cm). The Cd(II) removal by FMS was regarded as non-activated chemisorption that occurred very rapidly (even at a low temperature) with a low magnitude of activation energy. Primary adsorption mechanism was surface precipitation. Cadmium precipitated in the primary (Cd,Ca)CO3 form with a calcite-type structure on the FMS surface. A crust of rhombohedral crystals on the substrate was observed by SEM. Freshwater mussel shells have the potential as a renewable adsorbent to remove cadmium from water.
中文翻译:

镉在生物文石壳衍生生物吸附剂上的吸附特征和机理:分批和色谱柱研究
碳酸钙(CaCO 3淡水贻贝壳(FMS)衍生的富集生物材料被用作无孔生物吸附剂,以研究水溶液中镉的吸附特性和机理。通过比较吸附前后的FMS特性以及各种吸附研究,提出了吸附机理。FMS生物吸附剂的特征在于使用氮吸附/解吸等温线,X射线衍射,具有能谱的扫描电子显微镜,傅立叶变换红外光谱和零电荷点。批处理实验的结果表明,FMS在pH高于4.0的溶液中对Cd(II)离子具有极好的亲和力。离子强度的增加导致吸附在FMS上的Cd(II)的数量大大减少。动力学研究表明,吸附过程在约60分钟时迅速达到平衡。FMS生物吸附剂具有如下的Langmuir最大吸附能力:10°C时为18.2 mg / g <30°C时为26.0 mg / g <50°C时为28.6 mg / g。Cd(II)吸附过程是不可逆的,自发的(-ΔG °),吸热(+ ΔH °)和更随机(+ ΔS °)。金属阳离子的选择顺序(mmol / g)依次为Pb 2+ > Cd 2+ > Cu 2+ > Cr 3+ > Zn 2+。对于色谱柱实验,在流速(9 mL / min),初始Cd(II)浓度(10 mg / L)和床高(5 cm)下,可获得最高的托马斯吸附容量(7.86 mg / g)。通过FMS去除Cd(II)被认为是非活化的化学吸附,它以极低的活化能非常迅速地发生(即使在低温下)。主要的吸附机理是表面沉淀。初级(Cd,Ca)CO 3中沉淀的镉在FMS表面形成方解石型结构。通过SEM观察到基板上的菱面体晶体的结壳。淡水贻贝壳具有作为可再生吸附剂的潜力,可以从水中去除镉。



























 京公网安备 11010802027423号
京公网安备 11010802027423号