当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical detection of Zn(II)-induced amyloid-β aggregation: Insights into aggregation mechanisms
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2018-12-01 , DOI: 10.1016/j.jelechem.2018.10.016 Elena V. Suprun , Sergey P. Radko , Sergey A. Kozin , Vladimir A. Mitkevich , Alexander A. Makarov
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2018-12-01 , DOI: 10.1016/j.jelechem.2018.10.016 Elena V. Suprun , Sergey P. Radko , Sergey A. Kozin , Vladimir A. Mitkevich , Alexander A. Makarov
|
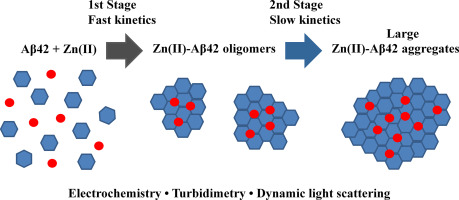
Abstract The tyrosine based electrochemical analysis of the kinetics of Zn(II)-induced aggregation of the 42-amino-acid-long amyloid-β peptide (Aβ42) implicated in Alzheimer's disease pathogenesis was carried out by square wave voltammetry on carbon screen printed electrodes. As previously reported, the Aβ42 electrooxidation peak current can serve as an estimate of the fraction of Aβ42 peptide molecules not included in Zn(II)-induced Aβ42 aggregates/oligomers. The current was found to drop in a Zn(II)-dependent manner prior to the first measurement (in a minute after the addition of Zn(II) ions) but to remain unchanged during the following 30-min incubation period. The electrochemical analysis was applied in parallel with turbidimetry and dynamic light scattering (DLS) to monitor in time the occurrence of Aβ42 aggregates induced by substoichiometric Zn(II) concentrations. In contrast to the current, Aβ42 solution turbidity steadily increased with time in a Zn(II)-dependent manner during the 30-min incubation period. The turbidity increase was accompanied by the occurrence of large (micron-sized) Aβ42 aggregates detected by DLS. These findings suggest that the Zn(II)-induced aggregation of Aβ42 molecules proceeds via a two-stage mechanism which includes as the first step a fast formation of Aβ42 oligomers (large enough to effectively suppress the Aβ42 electrooxidation via a tyrosine residue), followed by the relatively slow assembly of these oligomers into bulky (micron-sized) aggregates. The Zn(II)-triggered Aβ42 oligomerization and, consequently, the overall Zn(II)-induced Aβ42 aggregation were found to depend on the solution ionic strength. When applied to N-truncated and phosphorylated Aβ42 isoforms, Aβ(6-42) and pS8-Aβ42, the combined analysis has revealed that their aggregation behavior differs from that of the Aβ42 peptide. While Zn(II)-triggered Aβ(6-42) oligomers demonstrated a higher propensity to assemble into micron-sized aggregates, compared to the Aβ42 peptide, Zn(II)-triggered pS8-Aβ42 oligomers lack the ability to form large aggregates. Thus, the direct electrochemistry when combined with methods allowing for detection of aggregates may provide deeper mechanistic insights into protein/peptide aggregation.
中文翻译:

Zn(II) 诱导的淀粉样蛋白-β 聚集的电化学检测:对聚集机制的洞察
摘要 通过方波伏安法在碳丝网印刷电极上对 Zn(II) 诱导的 42 个氨基酸长的淀粉样蛋白 β 肽 (Aβ42) 聚集与阿尔茨海默病发病机制相关的动力学进行了基于酪氨酸的电化学分析。 . 如先前报道,Aβ42 电氧化峰值电流可作为不包含在 Zn(II) 诱导的 Aβ42 聚集体/寡聚体中的 Aβ42 肽分子分数的估计值。发现电流在第一次测量之前(在添加 Zn(II) 离子后一分钟内)以依赖于 Zn(II) 的方式下降,但在接下来的 30 分钟潜伏期内保持不变。电化学分析与浊度法和动态光散射 (DLS) 并行应用,以及时监测亚化学计量 Zn(II) 浓度诱导的 Aβ42 聚集体的发生。与目前相比,Aβ42 溶液的浊度在 30 分钟的潜伏期内以 Zn(II) 依赖性方式随时间稳定增加。浊度增加伴随着 DLS 检测到的大(微米级)Aβ42 聚集体的出现。这些发现表明,Zn(II) 诱导的 Aβ42 分子聚集通过两阶段机制进行,其中第一步是快速形成 Aβ42 寡聚体(足够大以通过酪氨酸残基有效抑制 Aβ42 电氧化),然后通过这些低聚物相对缓慢地组装成庞大的(微米级)聚集体。发现 Zn(II) 引发的 Aβ42 低聚和因此,整体 Zn(II) 诱导的 Aβ42 聚集取决于溶液离子强度。当应用于 N 截断和磷酸化的 Aβ42 同种型 Aβ(6-42) 和 pS8-Aβ42 时,联合分析表明它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。发现整体 Zn(II) 诱导的 Aβ42 聚集取决于溶液离子强度。当应用于 N 截断和磷酸化的 Aβ42 同种型 Aβ(6-42) 和 pS8-Aβ42 时,联合分析表明它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。发现整体 Zn(II) 诱导的 Aβ42 聚集取决于溶液离子强度。当应用于 N 截断和磷酸化的 Aβ42 同种型 Aβ(6-42) 和 pS8-Aβ42 时,联合分析表明它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。综合分析表明,它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。综合分析表明,它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。
更新日期:2018-12-01
中文翻译:

Zn(II) 诱导的淀粉样蛋白-β 聚集的电化学检测:对聚集机制的洞察
摘要 通过方波伏安法在碳丝网印刷电极上对 Zn(II) 诱导的 42 个氨基酸长的淀粉样蛋白 β 肽 (Aβ42) 聚集与阿尔茨海默病发病机制相关的动力学进行了基于酪氨酸的电化学分析。 . 如先前报道,Aβ42 电氧化峰值电流可作为不包含在 Zn(II) 诱导的 Aβ42 聚集体/寡聚体中的 Aβ42 肽分子分数的估计值。发现电流在第一次测量之前(在添加 Zn(II) 离子后一分钟内)以依赖于 Zn(II) 的方式下降,但在接下来的 30 分钟潜伏期内保持不变。电化学分析与浊度法和动态光散射 (DLS) 并行应用,以及时监测亚化学计量 Zn(II) 浓度诱导的 Aβ42 聚集体的发生。与目前相比,Aβ42 溶液的浊度在 30 分钟的潜伏期内以 Zn(II) 依赖性方式随时间稳定增加。浊度增加伴随着 DLS 检测到的大(微米级)Aβ42 聚集体的出现。这些发现表明,Zn(II) 诱导的 Aβ42 分子聚集通过两阶段机制进行,其中第一步是快速形成 Aβ42 寡聚体(足够大以通过酪氨酸残基有效抑制 Aβ42 电氧化),然后通过这些低聚物相对缓慢地组装成庞大的(微米级)聚集体。发现 Zn(II) 引发的 Aβ42 低聚和因此,整体 Zn(II) 诱导的 Aβ42 聚集取决于溶液离子强度。当应用于 N 截断和磷酸化的 Aβ42 同种型 Aβ(6-42) 和 pS8-Aβ42 时,联合分析表明它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。发现整体 Zn(II) 诱导的 Aβ42 聚集取决于溶液离子强度。当应用于 N 截断和磷酸化的 Aβ42 同种型 Aβ(6-42) 和 pS8-Aβ42 时,联合分析表明它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。发现整体 Zn(II) 诱导的 Aβ42 聚集取决于溶液离子强度。当应用于 N 截断和磷酸化的 Aβ42 同种型 Aβ(6-42) 和 pS8-Aβ42 时,联合分析表明它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。综合分析表明,它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。综合分析表明,它们的聚集行为不同于 Aβ42 肽的聚集行为。虽然与 Aβ42 肽相比,Zn(II) 触发的 Aβ(6-42) 寡聚体表现出更高的组装成微米级聚集体的倾向,但 Zn(II) 触发的 pS8-Aβ42 寡聚体缺乏形成大聚集体的能力。因此,当与允许检测聚集体的方法结合时,直接电化学可以提供对蛋白质/肽聚集的更深入的机械洞察。


























 京公网安备 11010802027423号
京公网安备 11010802027423号