当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism for O-O bond formation via radical coupling of metal and ligand based radicals – a new pathway
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-08 , DOI: 10.1021/jacs.8b06836 Yulia Pushkar 1 , Yuliana Pineda-Galvan 1 , Alireza K. Ravari 1 , Tatiana Otroshchenko 2 , Daniel A. Hartzler 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-08 , DOI: 10.1021/jacs.8b06836 Yulia Pushkar 1 , Yuliana Pineda-Galvan 1 , Alireza K. Ravari 1 , Tatiana Otroshchenko 2 , Daniel A. Hartzler 1
Affiliation
|
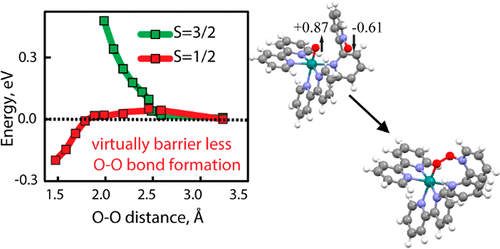
Artificial photosynthesis carries promise to deliver abundant clean energy for the needs of a growing population. Deep mechanistic understanding is required to achieve rational design of fast and durable water oxidation catalysts. Here we provided first evidence for a new mechanism of the O-O bond formation via radical coupling of the oxidized metal═oxo of radicaloid character (RuIV═O) and ligand based radical ([ligand-NO]+• cation radical). O-O bond formation is facilitated via spin alignment and takes place via a virtually barrier less pathway inside the single metal complex. In situ reactive intermediate conversion was monitored by mass spectrometry, resonance Raman (RR) and EPR. Computational analysis have shown that the formation of [ligand-NO]+• happens at a lower overpotential than the formation of the [RuV═O(ligand)]3+ intermediate. Overall, the presented paradigm for O-O bond formation opens new opportunities for rational catalyst design.
中文翻译:

通过金属和配体基自由基的自由基偶联形成OO键的机制——一种新途径
人工光合作用有望为不断增长的人口提供丰富的清洁能源。实现快速耐用的水氧化催化剂的合理设计需要深入的机理理解。在这里,我们提供了通过自由基耦合的氧化金属 = 氧自由基(RuIV = O)和基于配体的自由基([配体-NO]+• 阳离子自由基)形成 OO 键的新机制的第一个证据。OO 键的形成是通过自旋排列促进的,并通过单金属复合物内的几乎无障碍途径发生。通过质谱、共振拉曼 (RR) 和 EPR 监测原位反应性中间体转化。计算分析表明,[配体-NO]+• 的形成发生在比[RuV=O(配体)]3+ 中间体的形成更低的过电位下。
更新日期:2018-10-08
中文翻译:

通过金属和配体基自由基的自由基偶联形成OO键的机制——一种新途径
人工光合作用有望为不断增长的人口提供丰富的清洁能源。实现快速耐用的水氧化催化剂的合理设计需要深入的机理理解。在这里,我们提供了通过自由基耦合的氧化金属 = 氧自由基(RuIV = O)和基于配体的自由基([配体-NO]+• 阳离子自由基)形成 OO 键的新机制的第一个证据。OO 键的形成是通过自旋排列促进的,并通过单金属复合物内的几乎无障碍途径发生。通过质谱、共振拉曼 (RR) 和 EPR 监测原位反应性中间体转化。计算分析表明,[配体-NO]+• 的形成发生在比[RuV=O(配体)]3+ 中间体的形成更低的过电位下。


























 京公网安备 11010802027423号
京公网安备 11010802027423号