当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic study of the ceria supported, re-catalyzed deoxydehydration of vicinal OH groups†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-10-08 00:00:00 , DOI: 10.1039/c8cy01782d Yongjie Xi 1, 2, 3, 4 , Wenqiang Yang 1, 2, 3, 4 , Salai Cheettu Ammal 1, 2, 3, 4 , Jochen Lauterbach 1, 2, 3, 4 , Yomaira Pagan-Torres 1, 5, 6, 7 , Andreas Heyden 1, 2, 3, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-10-08 00:00:00 , DOI: 10.1039/c8cy01782d Yongjie Xi 1, 2, 3, 4 , Wenqiang Yang 1, 2, 3, 4 , Salai Cheettu Ammal 1, 2, 3, 4 , Jochen Lauterbach 1, 2, 3, 4 , Yomaira Pagan-Torres 1, 5, 6, 7 , Andreas Heyden 1, 2, 3, 4
Affiliation
|
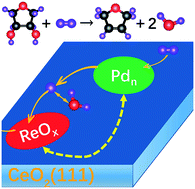
Deoxydehydration (DODH) is an emerging biomass deoxygenation process whereby vicinal OH groups are removed. Based on DFT calculations and microkinetic modeling, we seek to understand the mechanism of the Re-catalyzed deoxydehydration supported on CeO2(111). In addition, we aim at understanding the promotional effect of Pd in a heterogeneous ReOx–Pd/CeO2 DODH catalyst system. We disentangle the contribution of the oxide support, the oxide-supported single ReOx species, and a co-adsorbed Pd promoter that has no direct interaction with the Re species. In the absence of a nearby Pd cluster, a Re site is able to reduce subsurface Ce-ions of a hydroxylated CeO2(111) surface, leading to a catalytically active Re +6 species. The effect of Pd is twofold: (i) Pd catalyzes the hydrogen dissociation and spillover onto CeO2, which is an indispensable process for the regeneration of the Re catalyst, and (ii) Pd adsorbed in close proximity to Re on CeO2(111) facilitates the oxidation of Re to a +7 oxidation state, which leads to an even more active Re species than the Re +6 site present in the absence of Pd. The latter promotional effect of Pd (and change in oxidation state of Re) disappears with increasing Pd–Re distance and in the presence of oxygen defects on the ceria support. Under these conditions, the ReOx–Pd/CeO2 catalyst system exhibits appreciable activity consistent with recent experiments. The established mechanism and role of various species in the catalyst system help to better understand the deoxydehydration catalysis. Also, the importance of the Re oxidation state and the identified oxidation state modification mechanisms suggest a new pathway for tuning the properties of metal-oxide supported catalysts.
中文翻译:

二氧化铈支持的邻位OH基团的再催化脱氧脱水的机理研究†
脱氧脱水(DODH)是一种新兴的生物质脱氧工艺,可去除邻近的OH基团。基于DFT计算和微动力学模型,我们试图了解CeO 2(111)上负载的再催化脱氧脱水的机理。此外,我们旨在了解Pd在非均质ReO x –Pd / CeO 2 DODH催化剂体系中的促进作用。我们解开了氧化物载体,氧化物支持的单一ReO x物种以及与Re物种没有直接相互作用的共吸附Pd促进剂的贡献。在不存在附近的Pd团簇的情况下,Re位点能够还原羟基化CeO 2的亚表面Ce离子(111)表面,导致具有催化活性的Re +6物种。Pd的作用是双重的:(i)Pd催化氢解离并溢出到CeO 2上,这是Re催化剂再生的必不可少的过程,并且(ii)Pd紧靠Re吸附在CeO 2上(111 )可促进Re氧化为+7氧化态,从而比没有Pd时存在的Re +6活性位点更具活性。Pd的后一种促进作用(以及Re氧化态的改变)随着Pd-Re距离的增加以及氧化铈载体上存在氧缺陷而消失。在这些条件下,ReO x –Pd / CeO 2催化剂体系显示出与最近的实验一致的明显活性。各种物种在催化剂体系中已建立的机理和作用有助于更好地理解脱氧脱水催化作用。同样,Re氧化态的重要性和确定的氧化态修饰机制为调节金属氧化物负载型催化剂的性能提供了一条新途径。
更新日期:2018-10-08
中文翻译:

二氧化铈支持的邻位OH基团的再催化脱氧脱水的机理研究†
脱氧脱水(DODH)是一种新兴的生物质脱氧工艺,可去除邻近的OH基团。基于DFT计算和微动力学模型,我们试图了解CeO 2(111)上负载的再催化脱氧脱水的机理。此外,我们旨在了解Pd在非均质ReO x –Pd / CeO 2 DODH催化剂体系中的促进作用。我们解开了氧化物载体,氧化物支持的单一ReO x物种以及与Re物种没有直接相互作用的共吸附Pd促进剂的贡献。在不存在附近的Pd团簇的情况下,Re位点能够还原羟基化CeO 2的亚表面Ce离子(111)表面,导致具有催化活性的Re +6物种。Pd的作用是双重的:(i)Pd催化氢解离并溢出到CeO 2上,这是Re催化剂再生的必不可少的过程,并且(ii)Pd紧靠Re吸附在CeO 2上(111 )可促进Re氧化为+7氧化态,从而比没有Pd时存在的Re +6活性位点更具活性。Pd的后一种促进作用(以及Re氧化态的改变)随着Pd-Re距离的增加以及氧化铈载体上存在氧缺陷而消失。在这些条件下,ReO x –Pd / CeO 2催化剂体系显示出与最近的实验一致的明显活性。各种物种在催化剂体系中已建立的机理和作用有助于更好地理解脱氧脱水催化作用。同样,Re氧化态的重要性和确定的氧化态修饰机制为调节金属氧化物负载型催化剂的性能提供了一条新途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号