当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two-dimensional nickel hydroxide nanosheets with high-content of nickel(III) species towards superior urea electro-oxidation
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.jelechem.2018.10.007 Dongsheng Wang , Siwen Liu , Qiuping Gan , Jianniao Tian , Tayirjan Taylor Isimjan , Xiulin Yang
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.jelechem.2018.10.007 Dongsheng Wang , Siwen Liu , Qiuping Gan , Jianniao Tian , Tayirjan Taylor Isimjan , Xiulin Yang
|
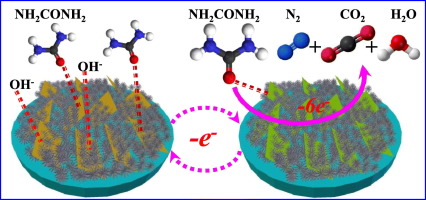
Abstract Development of high-efficient and stable electrocatalysts for urea oxidation reaction (UOR) is of a great challenge due to the sluggish kinetics of 6e− transfer process. Here, we have developed a facile and easy-to-scale approach to fabricate two-dimensional Ni(III)-rich Ni(OH)2 nanosheets on amine-functionalized carbon (Ni3+-rich Ni(OH)2/C-NH2). Morphological characterizations confirm the existence of nanosheets, and XPS spectra indicate that the content of Ni3+ species in Ni3+-rich Ni(OH)2/C-NH2 (ca. 57.6%) is significantly higher than that of in Ni(OH)2/C-NH2 (ca. 43.1%) and Ni(OH)2/C (ca. 20.7%). Electrochemical analyses illustrate that the as-prepared Ni3+-rich Ni(OH)2/C-NH2 catalyst exhibits the highest current density (91.72 mA cm−2) at a potential of 0.61 V, which is 2.06-, 2.08- and 3.47-fold higher than that of Ni(OH)2/C-NH2, Ni(OH)2/C and Pt/C, respectively. Moreover, the Ni3+-rich Ni(OH)2/C-NH2 catalyst also demonstrates an outstanding voltammetric cycles and long-term chronoamperometric stability. The superior electrocatalytic activity and stability could be ascribed to the synergistic effect of Ni3+ doping as well as the amine-functionalized carbon, where higher concentration Ni3+ species in Ni(OH)2 sheets could provide more active sites for adsorption and transformation of urea molecules, while fluffy C-NH2 support could enhance the ability of solute diffusion, electron transport and gas emissions, thereby dramatically improve the catalytic activity.
中文翻译:

具有高镍 (III) 物种含量的二维氢氧化镍纳米片可实现优异的尿素电氧化
摘要 由于 6e− 转移过程的动力学缓慢,开发用于尿素氧化反应 (UOR) 的高效稳定电催化剂是一个巨大的挑战。在这里,我们开发了一种简便且易于扩展的方法来在胺功能化碳(富含 Ni3+ 的 Ni(OH)2/C-NH2)上制造二维富含 Ni(III) 的 Ni(OH)2 纳米片. 形态表征证实了纳米片的存在,XPS 光谱表明富含 Ni3+ 的 Ni(OH)2/C-NH2(约 57.6%)中 Ni3+ 物种的含量显着高于 Ni(OH)2/ C-NH2(约 43.1%)和 Ni(OH)2/C(约 20.7%)。电化学分析表明,所制备的富含 Ni3+ 的 Ni(OH)2/C-NH2 催化剂在 0.61 V 的电位下表现出最高的电流密度 (91.72 mA cm-2),分别为 2.06-、2.08- 和 3.47-比 Ni(OH)2/C-NH2 高一倍,分别为 Ni(OH)2/C 和 Pt/C。此外,富含 Ni3+ 的 Ni(OH)2/C-NH2 催化剂还表现出出色的伏安循环和长期计时电流稳定性。优异的电催化活性和稳定性可归因于 Ni3+ 掺杂和胺功能化碳的协同作用,其中 Ni(OH)2 片中较高浓度的 Ni3+ 物种可以为尿素分子的吸附和转化提供更多的活性位点,而蓬松的C-NH2载体可以增强溶质扩散、电子传输和气体排放的能力,从而显着提高催化活性。
更新日期:2018-11-01
中文翻译:

具有高镍 (III) 物种含量的二维氢氧化镍纳米片可实现优异的尿素电氧化
摘要 由于 6e− 转移过程的动力学缓慢,开发用于尿素氧化反应 (UOR) 的高效稳定电催化剂是一个巨大的挑战。在这里,我们开发了一种简便且易于扩展的方法来在胺功能化碳(富含 Ni3+ 的 Ni(OH)2/C-NH2)上制造二维富含 Ni(III) 的 Ni(OH)2 纳米片. 形态表征证实了纳米片的存在,XPS 光谱表明富含 Ni3+ 的 Ni(OH)2/C-NH2(约 57.6%)中 Ni3+ 物种的含量显着高于 Ni(OH)2/ C-NH2(约 43.1%)和 Ni(OH)2/C(约 20.7%)。电化学分析表明,所制备的富含 Ni3+ 的 Ni(OH)2/C-NH2 催化剂在 0.61 V 的电位下表现出最高的电流密度 (91.72 mA cm-2),分别为 2.06-、2.08- 和 3.47-比 Ni(OH)2/C-NH2 高一倍,分别为 Ni(OH)2/C 和 Pt/C。此外,富含 Ni3+ 的 Ni(OH)2/C-NH2 催化剂还表现出出色的伏安循环和长期计时电流稳定性。优异的电催化活性和稳定性可归因于 Ni3+ 掺杂和胺功能化碳的协同作用,其中 Ni(OH)2 片中较高浓度的 Ni3+ 物种可以为尿素分子的吸附和转化提供更多的活性位点,而蓬松的C-NH2载体可以增强溶质扩散、电子传输和气体排放的能力,从而显着提高催化活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号