当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fully Conjugated [4]Chrysaorene. Redox-Coupled Anion Binding in a Tetraradicaloid Macrocycle
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-05 , DOI: 10.1021/jacs.8b09385 Hanna Gregolińska 1 , Marcin Majewski 1 , Piotr J. Chmielewski 1 , Janusz Gregoliński 1 , Alan Chien 2 , Jiawang Zhou 3 , Yi-Lin Wu 3 , Youn Jue Bae 3 , Michael R. Wasielewski 3 , Paul M. Zimmerman 2 , Marcin Stępień 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-05 , DOI: 10.1021/jacs.8b09385 Hanna Gregolińska 1 , Marcin Majewski 1 , Piotr J. Chmielewski 1 , Janusz Gregoliński 1 , Alan Chien 2 , Jiawang Zhou 3 , Yi-Lin Wu 3 , Youn Jue Bae 3 , Michael R. Wasielewski 3 , Paul M. Zimmerman 2 , Marcin Stępień 1
Affiliation
|
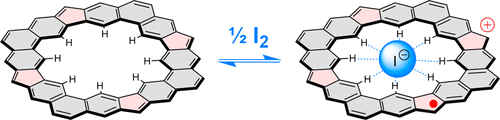
[4]Chrysaorene, a fully conjugated carbocyclic coronoid, is shown to be a low-bandgap π-conjugated system with a distinct open-shell character. The system shows good chemical stability and can be oxidized to well-defined radical cation and dication states. The cavity of [4]chrysaorene acts as an anion receptor toward halide ions with a particular selectivity toward iodides ( Ka = 207 ± 6 M-1). The interplay between anion binding and redox chemistry is demonstrated using a 1H NMR analysis in solution. In particular, a well-resolved, paramagnetically shifted spectrum of the [4]chrysaorene radical cation is observed, providing evidence for the inner binding of the iodide. The radical cation-iodide adduct can be generated in thin solid films of [4] chrysaorene by simple exposure to diiodine vapor.
中文翻译:

完全共轭 [4]Chrysaorene。四自由基大环中的氧化还原偶联阴离子结合
[4] Chrysaorene 是一种完全共轭的碳环类冠酮,被证明是一种低带隙 π 共轭系统,具有独特的开壳特性。该系统显示出良好的化学稳定性,可以氧化成明确定义的自由基阳离子和双阳离子状态。[4]chrysaorene 的空腔充当对卤化物离子的阴离子受体,对碘化物具有特定的选择性 (Ka = 207 ± 6 M-1)。使用溶液中的 1H NMR 分析证明了阴离子结合和氧化还原化学之间的相互作用。特别是,观察到 [4] 菊烯自由基阳离子的解析良好的顺磁位移光谱,为碘化物的内部结合提供了证据。通过简单地暴露于二碘蒸气中,可以在 [4] 菊烯的固体薄膜中生成自由基阳离子碘化物加合物。
更新日期:2018-10-05
中文翻译:

完全共轭 [4]Chrysaorene。四自由基大环中的氧化还原偶联阴离子结合
[4] Chrysaorene 是一种完全共轭的碳环类冠酮,被证明是一种低带隙 π 共轭系统,具有独特的开壳特性。该系统显示出良好的化学稳定性,可以氧化成明确定义的自由基阳离子和双阳离子状态。[4]chrysaorene 的空腔充当对卤化物离子的阴离子受体,对碘化物具有特定的选择性 (Ka = 207 ± 6 M-1)。使用溶液中的 1H NMR 分析证明了阴离子结合和氧化还原化学之间的相互作用。特别是,观察到 [4] 菊烯自由基阳离子的解析良好的顺磁位移光谱,为碘化物的内部结合提供了证据。通过简单地暴露于二碘蒸气中,可以在 [4] 菊烯的固体薄膜中生成自由基阳离子碘化物加合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号