当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Diverted Aerobic Heck Reaction Enables Selective 1,3-Diene and 1,3,5-Triene Synthesis Through C−C Bond Scission
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-05 , DOI: 10.1021/jacs.8b10007 Neil J McAlpine 1 , Long Wang 1 , Brad P Carrow 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-05 , DOI: 10.1021/jacs.8b10007 Neil J McAlpine 1 , Long Wang 1 , Brad P Carrow 1
Affiliation
|
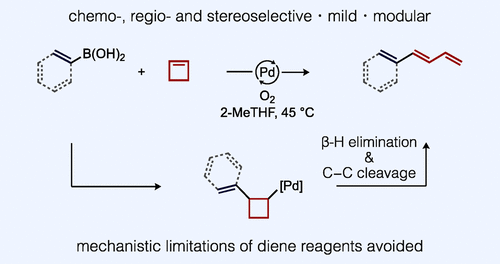
Substituted 1,3-dienes are valuable synthetic intermediates used in myriad catalytic transformations, yet modern catalytic methods for their preparation in a highly modular fashion using simple precursors are relatively few. We report here an aerobic boron Heck reaction with cyclobutene that forms exclusively linear 1-aryl-1,3-dienes using (hetero)arylboronic acids, or 1,3,5-trienes using alkenylboronic acids, rather than typical Heck products (i.e., substituted cyclobutenes). Experimental and computational mechanistic data support a pericyclic mechanism for C-C bond cleavage that enables the cycloalkene to circumvent established limitations associated with diene reagents in Heck-type reactions.
中文翻译:

转移有氧 Heck 反应通过 C−C 键断裂选择性合成 1,3-二烯和 1,3,5-三烯
取代的 1,3-二烯是用于无数催化转化的有价值的合成中间体,但使用简单前体以高度模块化方式制备它们的现代催化方法相对较少。我们在此报告了与环丁烯的需氧硼 Heck 反应,该反应使用(杂)芳基硼酸形成专门的线性 1-芳基-1,3-二烯,或使用烯基硼酸形成 1,3,5-三烯,而不是典型的 Heck 产物(即取代的环丁烯)。实验和计算机理数据支持 CC 键断裂的周环机制,该机制使环烯烃能够规避 Heck 型反应中与二烯试剂相关的既定限制。
更新日期:2018-10-05
中文翻译:

转移有氧 Heck 反应通过 C−C 键断裂选择性合成 1,3-二烯和 1,3,5-三烯
取代的 1,3-二烯是用于无数催化转化的有价值的合成中间体,但使用简单前体以高度模块化方式制备它们的现代催化方法相对较少。我们在此报告了与环丁烯的需氧硼 Heck 反应,该反应使用(杂)芳基硼酸形成专门的线性 1-芳基-1,3-二烯,或使用烯基硼酸形成 1,3,5-三烯,而不是典型的 Heck 产物(即取代的环丁烯)。实验和计算机理数据支持 CC 键断裂的周环机制,该机制使环烯烃能够规避 Heck 型反应中与二烯试剂相关的既定限制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号