当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Mechanism of ATP Hydrolysis in an ABC Transporter
ACS Central Science ( IF 18.2 ) Pub Date : 2018-10-05 00:00:00 , DOI: 10.1021/acscentsci.8b00369 Marten Prieß 1 , Hendrik Göddeke 1 , Gerrit Groenhof 2 , Lars V. Schäfer 1
ACS Central Science ( IF 18.2 ) Pub Date : 2018-10-05 00:00:00 , DOI: 10.1021/acscentsci.8b00369 Marten Prieß 1 , Hendrik Göddeke 1 , Gerrit Groenhof 2 , Lars V. Schäfer 1
Affiliation
|
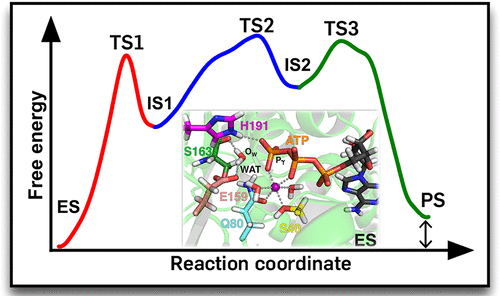
Hydrolysis of nucleoside triphosphate (NTP) plays a key role for the function of many biomolecular systems. However, the chemistry of the catalytic reaction in terms of an atomic-level understanding of the structural, dynamic, and free energy changes associated with it often remains unknown. Here, we report the molecular mechanism of adenosine triphosphate (ATP) hydrolysis in the ATP-binding cassette (ABC) transporter BtuCD-F. Free energy profiles obtained from hybrid quantum mechanical/molecular mechanical (QM/MM) molecular dynamics (MD) simulations show that the hydrolysis reaction proceeds in a stepwise manner. First, nucleophilic attack of an activated lytic water molecule at the ATP γ-phosphate yields ADP + HPO42– as intermediate product. A conserved glutamate that is located very close to the γ-phosphate transiently accepts a proton and thus acts as catalytic base. In the second step, the proton is transferred back from the catalytic base to the γ-phosphate, yielding ADP + H2PO4–. These two chemical reaction steps are followed by rearrangements of the hydrogen bond network and the coordination of the Mg2+ ion. The rate constant estimated from the computed free energy barriers is in very good agreement with experiments. The overall free energy change of the reaction is close to zero, suggesting that phosphate bond cleavage itself does not provide a power stroke for conformational changes. Instead, ATP binding is essential for tight dimerization of the nucleotide-binding domains and the transition of the transmembrane domains from inward- to outward-facing, whereas ATP hydrolysis resets the conformational cycle. The mechanism is likely relevant for all ABC transporters and might have implications also for other NTPases, as many residues involved in nucleotide binding and hydrolysis are strictly conserved.
中文翻译:

ABC转运蛋白中ATP水解的分子机理
三磷酸核苷(NTP)的水解对于许多生物分子系统的功能起着关键作用。然而,就原子级理解与之相关的结构,动态和自由能变化而言,催化反应的化学性质仍然是未知的。在这里,我们报告在ATP结合盒(ABC)转运蛋白BtuCD-F中三磷酸腺苷(ATP)水解的分子机制。从混合量子力学/分子力学(QM / MM)分子动力学(MD)模拟获得的自由能曲线表明,水解反应是以逐步方式进行的。首先,活化的裂解水分子在ATPγ-磷酸盐处的亲核攻击产生ADP + HPO 4 2–作为中间产品。非常靠近γ-磷酸的保守的谷氨酸盐瞬时接受质子,因此起催化碱的作用。在第二步骤中,质子转移从催化基部到γ-磷酸盐背面,产生ADP + H 2 PO 4 - 。这两个化学反应步骤之后是氢键网络的重排和Mg 2+的配位离子。根据计算出的自由能垒估计的速率常数与实验非常吻合。反应的总自由能变化接近于零,表明磷酸酯键断裂本身不提供构象变化的功率冲程。取而代之的是,ATP结合对于核苷酸结合结构域的紧密二聚化和跨膜结构域从向内到向外的过渡至关重要,而ATP水解会重置构象循环。该机制可能与所有ABC转运蛋白有关,并且可能对其他NTP酶也有影响,因为严格限制了核苷酸结合和水解中涉及的许多残基。
更新日期:2018-10-05
中文翻译:

ABC转运蛋白中ATP水解的分子机理
三磷酸核苷(NTP)的水解对于许多生物分子系统的功能起着关键作用。然而,就原子级理解与之相关的结构,动态和自由能变化而言,催化反应的化学性质仍然是未知的。在这里,我们报告在ATP结合盒(ABC)转运蛋白BtuCD-F中三磷酸腺苷(ATP)水解的分子机制。从混合量子力学/分子力学(QM / MM)分子动力学(MD)模拟获得的自由能曲线表明,水解反应是以逐步方式进行的。首先,活化的裂解水分子在ATPγ-磷酸盐处的亲核攻击产生ADP + HPO 4 2–作为中间产品。非常靠近γ-磷酸的保守的谷氨酸盐瞬时接受质子,因此起催化碱的作用。在第二步骤中,质子转移从催化基部到γ-磷酸盐背面,产生ADP + H 2 PO 4 - 。这两个化学反应步骤之后是氢键网络的重排和Mg 2+的配位离子。根据计算出的自由能垒估计的速率常数与实验非常吻合。反应的总自由能变化接近于零,表明磷酸酯键断裂本身不提供构象变化的功率冲程。取而代之的是,ATP结合对于核苷酸结合结构域的紧密二聚化和跨膜结构域从向内到向外的过渡至关重要,而ATP水解会重置构象循环。该机制可能与所有ABC转运蛋白有关,并且可能对其他NTP酶也有影响,因为严格限制了核苷酸结合和水解中涉及的许多残基。


























 京公网安备 11010802027423号
京公网安备 11010802027423号