Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2018-10-04 , DOI: 10.1016/j.jfluchem.2018.10.001 Oleg V. Stanko , Yuliya V. Rassukana , Kateryna A. Zamulko , Viktoriya V. Dyakonenko , Svitlana V. Shishkina , Petro P. Onys’ko
|
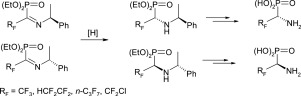
The asymmetric synthesis of biorelevant aminophosphonates and aminophosphonic acids bearing trifluoromethyl, tetrafluoroethyl, perfluoropropyl or chlorodifluoromethyl group in the α-position to phosphonyl group is developed. Diastereoselective reduction of α-(poly)fluoroalkylated iminophosphonates, bearing stereodirecting α-phenylethyl group at the nitrogen atom, with sodium borohydride produces diastereomeric N-(α-phenylethyl) polyfluoroalkylphosphonates, readily separated by column chromatography. The highly stereoselective reaction of (R)-N-tert-butylsulfinylimine of tetrafluoropropanal with generated in situ diethyltrimethysilylphosphite leads to (R,R)-N-(tert-butylsulfinyl)tetrafluopropylphosphonate. Sequential N- and O-deprotection affords enantiomerically pure polyfluoroalkylphosphonate and aminophosphonic acids. The presence of α-phenylethyl group at the nitrogen atom allows easy determination of absolute configuration of the newly formed stereogenic center by the 31P,19F NMR method.
中文翻译:

非对映选择性合成多氟烷基化的α-氨基膦酸衍生物
开发了在膦酰基的α位带有三氟甲基,四氟乙基,全氟丙基或氯二氟甲基的生物相关氨基膦酸酯和氨基膦酸的不对称合成。用氢硼化钠将在氮原子上带有立体定向α-苯乙基的α-(多)氟烷基化亚氨基膦酸酯进行非对映选择性还原,生成非对映体N-(α-苯基乙基)多氟烷基膦酸酯,可通过柱色谱法轻松分离。四氟丙醛的(R)-N-叔丁基亚磺酰亚胺与原位生成的二乙基三甲基甲硅烷基亚磷酸酯的高度立体选择性反应导致(R,R)-N-(叔丁基亚磺酰基)四氟丙基膦酸酯。顺序的N-和O-脱保护得到对映体纯的多氟烷基膦酸酯和氨基膦酸。氮原子上存在α-苯乙基,可通过31 P,19 F NMR方法轻松确定新形成的立体异构中心的绝对构型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号