当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Selective Palladium-Catalyzed Hydroborylative Carbocyclization of Bisallenes to Seven-Membered Rings
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-03 , DOI: 10.1021/jacs.8b08708 Can Zhu 1 , Bin Yang 1 , Binh Khanh Mai 1 , Sara Palazzotto 1 , Youai Qiu 1 , Arnar Gudmundsson 1 , Alexander Ricke 1 , Fahmi Himo 1 , Jan-E. Bäckvall 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-03 , DOI: 10.1021/jacs.8b08708 Can Zhu 1 , Bin Yang 1 , Binh Khanh Mai 1 , Sara Palazzotto 1 , Youai Qiu 1 , Arnar Gudmundsson 1 , Alexander Ricke 1 , Fahmi Himo 1 , Jan-E. Bäckvall 1
Affiliation
|
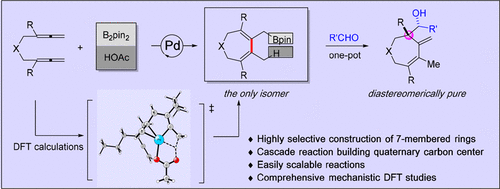
A highly selective palladium-catalyzed hydroborylative carbocyclization of bisallenes to afford seven-membered rings has been established. This ring-closing coupling reaction showed good functional group compatibility with high chemo- and regioselectivity, as seven-membered ring 3 was the only product obtained. The extensive use of different linkers, including nitrogen, oxygen, malononitrile, and malonate, showed a broad substrate scope for this approach. A one-pot cascade reaction was realized by trapping the primary allylboron compound with an aldehyde, affording a diastereomerically pure alcohol and a quaternary carbon center by formation of a new C-C bond. A comprehensive mechanistic DFT investigation is also presented. The calculations suggest that the reaction proceeds via a concerted hydropalladation pathway from a Pd(0)-olefin complex rather than via a pathway involving a defined palladium hydride species. The reaction was significantly accelerated by the coordination of the pendant olefin, as well as the introduction of suitable substituents in the bridge, due to the Thorpe-Ingold effect.
中文翻译:

高选择性钯催化氢硼化碳环化双丙二烯为七元环
已经建立了高选择性钯催化的双丙二烯的硼氢化碳环化以提供七元环。这种闭环偶联反应显示出良好的官能团兼容性和高化学和区域选择性,因为七元环 3 是唯一获得的产物。不同接头的广泛使用,包括氮、氧、丙二腈和丙二酸,表明该方法具有广泛的底物范围。通过用醛捕获主要的烯丙基硼化合物实现一锅级联反应,通过形成新的 CC 键提供非对映异构纯醇和季碳中心。还介绍了全面的机械 DFT 调查。计算表明,该反应通过来自 Pd(0)-烯烃络合物的协同氢化钯化途径进行,而不是通过涉及特定氢化钯物质的途径进行。由于 Thorpe-Ingold 效应,侧链烯烃的配位以及在桥中引入合适的取代基显着加速了反应。
更新日期:2018-10-03
中文翻译:

高选择性钯催化氢硼化碳环化双丙二烯为七元环
已经建立了高选择性钯催化的双丙二烯的硼氢化碳环化以提供七元环。这种闭环偶联反应显示出良好的官能团兼容性和高化学和区域选择性,因为七元环 3 是唯一获得的产物。不同接头的广泛使用,包括氮、氧、丙二腈和丙二酸,表明该方法具有广泛的底物范围。通过用醛捕获主要的烯丙基硼化合物实现一锅级联反应,通过形成新的 CC 键提供非对映异构纯醇和季碳中心。还介绍了全面的机械 DFT 调查。计算表明,该反应通过来自 Pd(0)-烯烃络合物的协同氢化钯化途径进行,而不是通过涉及特定氢化钯物质的途径进行。由于 Thorpe-Ingold 效应,侧链烯烃的配位以及在桥中引入合适的取代基显着加速了反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号