当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Queueing Theory-Based Perspective of the Kinetics of “Channeled” Enzyme Cascade Reactions
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1021/acscatal.8b02760 Stanislav Tsitkov 1 , Theo Pesenti 1, 2 , Henri Palacci 1 , Jose Blanchet 3 , Henry Hess 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1021/acscatal.8b02760 Stanislav Tsitkov 1 , Theo Pesenti 1, 2 , Henri Palacci 1 , Jose Blanchet 3 , Henry Hess 1
Affiliation
|
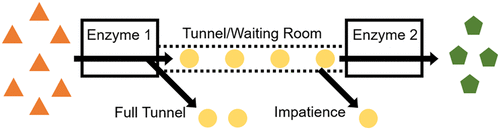
Queueing approaches can capture the stochastic dynamics of chemical reactions and provide a more accurate picture of the reaction kinetics than coupled differential equations in situations where the number of molecules is small. A striking example of such a situation is an enzyme cascade with substrate channeling, where reaction intermediates are directly passed from one enzyme to the next via tunnels or surface paths with limited capacity. In order to better understand the contribution of the stochastic dynamics to the observed enhancement in cascade throughput as a result from substrate channeling, we compare the results of a model using differential equations to describe concentration changes with a queueing model. The continuum model and the queueing model yield identical results, except when the maximum rate of reaction of the enzymes are similar. In two enzyme cascades, the queueing model predicts at most a 50% smaller throughput than the continuum model even if the waiting room size (the maximum number of molecules that can fit in the tunnel or surface path between enzymes) is limited to only one molecule and the enzymes are perfectly matched in their kinetic rates. In longer cascades, the discrepancy increases, reaching a 5-fold difference for a 10 enzyme cascade. In line with theoretical results from queueing theory, stochastic effects are found to always reduce cascade throughput, which means they cannot contribute to the experimentally observed enhancement in throughput due to channeling.
中文翻译:

基于排队论的“通道化”酶级联反应动力学的观点
排队方法可以捕获化学反应的随机动力学,并在分子数量少的情况下提供比耦合微分方程更准确的反应动力学图。这种情况的一个显着例子是具有底物通道的酶级联反应,其中反应中间体通过通道或表面路径以有限的能力直接从一种酶直接传递至另一种酶。为了更好地理解随机动力学对底物通道化导致的级联通量提高的影响,我们使用微分方程比较了模型的结果,以排队模型描述了浓度变化。连续模型和排队模型得出相同的结果,酶的最大反应速率相似时除外。在两个酶级联反应中,即使候诊室大小(可容纳在酶之间的通道或表面路径中的最大分子数)仅限于一个分子,排队模型预测的吞吐量最多比连续模型小50%。而且这些酶的动力学速率完全匹配。在较长的级联反应中,差异会增加,对于10个酶的级联反应,差异会达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。排队模型预测的吞吐量最多比连续模型小50%,即使等候室的大小(可容纳在酶之间的通道或表面路径中的最大分子数)仅限于一个分子且酶是完美的动力学速率相匹配。在较长的级联反应中,差异会增加,对于10个酶的级联反应,差异会达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。排队模型预测的吞吐量最多比连续模型小50%,即使等候室的大小(可容纳在酶之间的通道或表面路径中的最大分子数)仅限于一个分子且酶是完美的动力学速率相匹配。在较长的级联反应中,差异会增加,对于10个酶的级联反应,差异会达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。差异增加,对于10个酶的级联,差异达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。差异增加,对于10个酶的级联,差异达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。
更新日期:2018-10-03
中文翻译:

基于排队论的“通道化”酶级联反应动力学的观点
排队方法可以捕获化学反应的随机动力学,并在分子数量少的情况下提供比耦合微分方程更准确的反应动力学图。这种情况的一个显着例子是具有底物通道的酶级联反应,其中反应中间体通过通道或表面路径以有限的能力直接从一种酶直接传递至另一种酶。为了更好地理解随机动力学对底物通道化导致的级联通量提高的影响,我们使用微分方程比较了模型的结果,以排队模型描述了浓度变化。连续模型和排队模型得出相同的结果,酶的最大反应速率相似时除外。在两个酶级联反应中,即使候诊室大小(可容纳在酶之间的通道或表面路径中的最大分子数)仅限于一个分子,排队模型预测的吞吐量最多比连续模型小50%。而且这些酶的动力学速率完全匹配。在较长的级联反应中,差异会增加,对于10个酶的级联反应,差异会达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。排队模型预测的吞吐量最多比连续模型小50%,即使等候室的大小(可容纳在酶之间的通道或表面路径中的最大分子数)仅限于一个分子且酶是完美的动力学速率相匹配。在较长的级联反应中,差异会增加,对于10个酶的级联反应,差异会达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。排队模型预测的吞吐量最多比连续模型小50%,即使等候室的大小(可容纳在酶之间的通道或表面路径中的最大分子数)仅限于一个分子且酶是完美的动力学速率相匹配。在较长的级联反应中,差异会增加,对于10个酶的级联反应,差异会达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。差异增加,对于10个酶的级联,差异达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。差异增加,对于10个酶的级联,差异达到5倍。与排队论的理论结果相一致,发现随机效应总是会降低级联吞吐量,这意味着它们无法通过信道实验帮助提高吞吐量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号