Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-10-03 , DOI: 10.1016/j.tetlet.2018.10.004 Prakash K. Warghude , Pankaj D. Dharpure , Ramakrishna G. Bhat
|
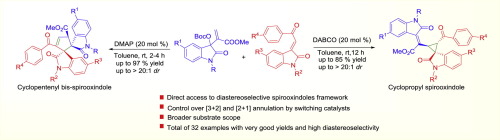
A highly diastereoselective organocatalytic synthesis of cyclopentenyl bis-spirooxindoles and cyclopropyl spirooxindoles has been achieved via [3 + 2] and [2 + 1] annulations. The tertiary amine catalysts have been effectively employed to tune the cycloaddition of isatin-derived MBH carbonates and 3-methyleneoxindoles for the outcome of two different spirooxindole frameworks with vicinal quaternary spiro centers and three contiguous stereocenters. The reactions worked under mild and practical conditions to afford the spirooxindoles in good to excellent yields with very high diastereoselectvity.
中文翻译:

伊斯兰衍生的MBH碳酸酯和3-亚甲基恶吲哚的环加成反应,以构建非对映选择性的环戊烯基双-螺氧并吲哚和环丙基螺氧并吲哚:催化剂控制的[3 + 2]和[2 + 1]环化反应
通过[3 + 2]和[2 +1]环化反应已实现了高度非对映选择性的有机催化合成环戊烯基双-螺氧并吲哚和环丙基螺氧并吲哚。叔胺催化剂已被有效地用于调节由靛红衍生的MBH碳酸酯和3-亚甲基吲哚的环加成反应,从而得到具有邻位季螺中心和三个连续的立体中心的两个不同的螺并吲哚骨架。该反应在温和且实际的条件下进行,从而以非常高的非对映选择性提供了良好至优异产率的螺硫辛多。


























 京公网安备 11010802027423号
京公网安备 11010802027423号