当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight into the Binding Profile of DCVJ and α-Synuclein Fibril Revealed by Multiscale Simulations.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-10-17 , DOI: 10.1021/acschemneuro.8b00465 Guanglin Kuang 1 , N Arul Murugan 1 , Hans Ågren 1, 2
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-10-17 , DOI: 10.1021/acschemneuro.8b00465 Guanglin Kuang 1 , N Arul Murugan 1 , Hans Ågren 1, 2
Affiliation
|
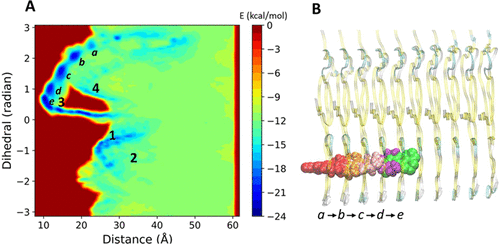
Parkinson's disease (PD) is a serious neurodegenerative disease and is characterized by abnormal α-synuclein (α-syn) accumulation in Lewy bodies (LB) and Lewy neurites (LN), which makes α-syn an important imaging target for PD. An imaging probe that quantifies fibrillar α-syn can enhance the clinical diagnosis of PD and can also be used to evaluate the efficacy of therapeutics aimed at reducing the abnormal aggregation of the α-syn fibril in the brain. In this paper, we study the binding profile of fibrillar α-syn with a fluorescent probe 4-(dicyanovinyl)julolidine (DCVJ), which is being explored for identifying α-syn imaging agents. A multiscale simulation workflow including molecular docking, molecular dynamics, metadynamics, and QM/MM calculations was implemented. We find that DCVJ can bind to multiple sites of α-syn which are located either at the surface or in the core. Free energy calculations using implicit solvent models reveal that the most favorable binding mode for DCVJ is associated with the core binding site and is further confirmed by metadyamics simulation. Besides, a dynamic binding pathway is discovered, which reveals that DCVJ binds gradually into the core of the fibril passing through several intermediate states. The conformational arrest of the dicyano vinyl group in the fibrillar environment could explain the reason behind the fibril-specific fluorescence of DCVJ. Furthermore, based on hybrid QM/MM calculations, the molecular geometry of the dicyano vinyl group is found to be environment specific which explains why DCVJ serves as a staining agent for such fibrillar-like environments. Our results could be helpful for elucidating the binding mechanism of imaging tracers with the fibrillar form of α-syn and explain their fibrillar-specific optical properties, a knowledge that in turn can be used to guide the design and development of compounds with higher affinity and selectivity for α-syn using structure-based strategies.
中文翻译:

通过多尺度模拟揭示了对DCVJ和α-突触核蛋白原纤维的结合谱的机械洞察力。
帕金森氏病(PD)是一种严重的神经退行性疾病,其特征在于路易体(LB)和路易神经突(LN)中异常的α-突触核蛋白(α-syn)蓄积,这使α-syn成为PD的重要成像靶标。量化原纤维α-syn的成像探针可以增强PD的临床诊断,也可以用于评估旨在减少脑中α-syn纤维异常聚集的治疗方法的功效。在本文中,我们研究了原纤维α-syn与荧光探针4-(双氰基乙烯基)julolidine(DCVJ)的结合情况,该探针正在探索中,以鉴定α-syn成像剂。实现了包括分子对接,分子动力学,元动力学和QM / MM计算在内的多尺度模拟工作流程。我们发现DCVJ可以结合到位于表面或核心的多个α-syn位点。使用隐式溶剂模型的自由能计算表明,DCVJ最有利的结合模式与核心结合位点相关,并通过后代动力学模拟得到了进一步证实。此外,发现了动态结合途径,其揭示DCVJ通过几个中间状态逐渐结合到原纤维的核心中。在纤维状环境中二氰基乙烯基的构象阻滞可以解释DCVJ的原纤维特异性荧光背后的原因。此外,基于混合QM / MM计算,发现二氰基乙烯基的分子几何结构是特定于环境的,这解释了为什么DCVJ用作此类原纤维状环境的染色剂。
更新日期:2018-10-02
中文翻译:

通过多尺度模拟揭示了对DCVJ和α-突触核蛋白原纤维的结合谱的机械洞察力。
帕金森氏病(PD)是一种严重的神经退行性疾病,其特征在于路易体(LB)和路易神经突(LN)中异常的α-突触核蛋白(α-syn)蓄积,这使α-syn成为PD的重要成像靶标。量化原纤维α-syn的成像探针可以增强PD的临床诊断,也可以用于评估旨在减少脑中α-syn纤维异常聚集的治疗方法的功效。在本文中,我们研究了原纤维α-syn与荧光探针4-(双氰基乙烯基)julolidine(DCVJ)的结合情况,该探针正在探索中,以鉴定α-syn成像剂。实现了包括分子对接,分子动力学,元动力学和QM / MM计算在内的多尺度模拟工作流程。我们发现DCVJ可以结合到位于表面或核心的多个α-syn位点。使用隐式溶剂模型的自由能计算表明,DCVJ最有利的结合模式与核心结合位点相关,并通过后代动力学模拟得到了进一步证实。此外,发现了动态结合途径,其揭示DCVJ通过几个中间状态逐渐结合到原纤维的核心中。在纤维状环境中二氰基乙烯基的构象阻滞可以解释DCVJ的原纤维特异性荧光背后的原因。此外,基于混合QM / MM计算,发现二氰基乙烯基的分子几何结构是特定于环境的,这解释了为什么DCVJ用作此类原纤维状环境的染色剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号