Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting Tumor Microenvironment by Bioreduction-Activated Nanoparticles for Light-Triggered Virotherapy
ACS Nano ( IF 17.1 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1021/acsnano.8b02813 S.-Ja Tseng,Ivan M. Kempson,Kuo-Yen Huang,Hsin-Jung Li,Yu-Chen Fa,Yi-Cheng Ho,Zi-Xian Liao,Pan-Chyr Yang
ACS Nano ( IF 17.1 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1021/acsnano.8b02813 S.-Ja Tseng,Ivan M. Kempson,Kuo-Yen Huang,Hsin-Jung Li,Yu-Chen Fa,Yi-Cheng Ho,Zi-Xian Liao,Pan-Chyr Yang
|
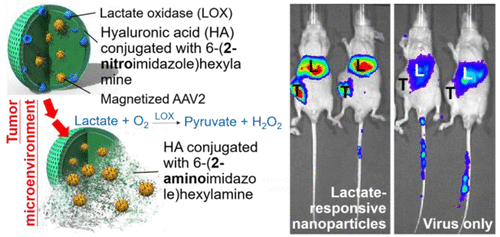
Solid tumors characteristically display higher levels of lactate production due to anaerobic metabolism of glucose. Meanwhile, the U.S. Food and Drug Administration (FDA) has approved virotherapy for use in cancer treatment; however systemic administration remains as a particular challenge. Here we report exploitation of tumor lactate production in designing a hypoxia-responsive carrier, self-assembled from hyaluronic acid (HA) conjugated with 6-(2-nitroimidazole)hexylamine, for localized release of recombinant adeno-associated virus serotype 2 (AAV2). The carrier is loaded with lactate oxidase (LOX) and is permeable to small molecules such as the lactate that accumulates in the tumor. Subsequently, LOX oxidizes the lactate to pyruvate inside the carrier, accompanied by internal lowering of oxygen partial pressure. Bioreduction of the 2-nitroimidazole of the HA conjugated with 6-(2-nitroimidazole)hexylamine converts it into a hydrophilic moiety and electrostatically dissociates the carrier and virus. Efficacious and specific delivery was proven by transduction of a photosensitive protein (KillerRed), enabling significant limitation in tumor growth in vivo with photodynamic therapy. An approximate 2.44-fold reduction in tumor weight was achieved after a 2-week course, compared with control groups. Furthermore, conjugation of the AAV2 with iron oxide nanoparticles (“magnetized” AAV2) facilitated magnetic resonance imaging tracking of the virus in vivo. Taken together, the solid tumor microenvironment promotes bioreduction of the lactate-responsive carrier, providing rapid and specific delivery of AAV2 for light-triggered virotherapy via systemic administration.
中文翻译:

通过生物还原激活的纳米粒子靶向肿瘤微环境的光触发病毒疗法。
由于葡萄糖的厌氧代谢,实体瘤通常表现出较高水平的乳酸产生。同时,美国食品和药物管理局(FDA)已批准病毒疗法用于癌症治疗;然而,系统管理仍然是一个特殊的挑战。在这里,我们报道了在设计低氧反应性载体中从肿瘤乳酸产生中的利用,该载体由与6-(2-硝基咪唑)己胺结合的透明质酸(HA)自组装而成,用于局部释放重组腺伴随病毒血清型2(AAV2) 。所述载体装载有乳酸氧化酶(LOX),并且对于诸如在肿瘤中累积的乳酸之类的小分子是可渗透的。随后,LOX在载体内部将乳酸氧化为丙酮酸,同时内部降低了氧分压。与6-(2-硝基咪唑)己胺缀合的HA的2-硝基咪唑的生物还原将其转化为亲水部分,并静电分离载体和病毒。通过光敏蛋白(KillerRed)的转导证明了有效而特异性的递送,从而显着限制了肿瘤的生长在体内进行光动力疗法。与对照组相比,在2周的疗程后,肿瘤重量减少了约2.44倍。此外,AAV2与氧化铁纳米颗粒(“磁化” AAV2)的结合促进了体内病毒的磁共振成像跟踪。总体而言,实体瘤微环境促进了乳酸反应性载体的生物还原,从而通过全身给药为光触发的病毒疗法提供了AAV2的快速和特异性递送。
更新日期:2018-10-02
中文翻译:

通过生物还原激活的纳米粒子靶向肿瘤微环境的光触发病毒疗法。
由于葡萄糖的厌氧代谢,实体瘤通常表现出较高水平的乳酸产生。同时,美国食品和药物管理局(FDA)已批准病毒疗法用于癌症治疗;然而,系统管理仍然是一个特殊的挑战。在这里,我们报道了在设计低氧反应性载体中从肿瘤乳酸产生中的利用,该载体由与6-(2-硝基咪唑)己胺结合的透明质酸(HA)自组装而成,用于局部释放重组腺伴随病毒血清型2(AAV2) 。所述载体装载有乳酸氧化酶(LOX),并且对于诸如在肿瘤中累积的乳酸之类的小分子是可渗透的。随后,LOX在载体内部将乳酸氧化为丙酮酸,同时内部降低了氧分压。与6-(2-硝基咪唑)己胺缀合的HA的2-硝基咪唑的生物还原将其转化为亲水部分,并静电分离载体和病毒。通过光敏蛋白(KillerRed)的转导证明了有效而特异性的递送,从而显着限制了肿瘤的生长在体内进行光动力疗法。与对照组相比,在2周的疗程后,肿瘤重量减少了约2.44倍。此外,AAV2与氧化铁纳米颗粒(“磁化” AAV2)的结合促进了体内病毒的磁共振成像跟踪。总体而言,实体瘤微环境促进了乳酸反应性载体的生物还原,从而通过全身给药为光触发的病毒疗法提供了AAV2的快速和特异性递送。


























 京公网安备 11010802027423号
京公网安备 11010802027423号