当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
SLC30A10 Mutation Involved in Parkinsonism Results in Manganese Accumulation within Nanovesicles of the Golgi Apparatus.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-10-15 , DOI: 10.1021/acschemneuro.8b00451 Asuncion Carmona 1, 2 , Charles E Zogzas 3 , Stéphane Roudeau 1, 2 , Francesco Porcaro 1, 2 , Jan Garrevoet 4 , Kathryn M Spiers 4 , Murielle Salomé 5 , Peter Cloetens 5 , Somshuvra Mukhopadhyay 3 , Richard Ortega 1, 2
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-10-15 , DOI: 10.1021/acschemneuro.8b00451 Asuncion Carmona 1, 2 , Charles E Zogzas 3 , Stéphane Roudeau 1, 2 , Francesco Porcaro 1, 2 , Jan Garrevoet 4 , Kathryn M Spiers 4 , Murielle Salomé 5 , Peter Cloetens 5 , Somshuvra Mukhopadhyay 3 , Richard Ortega 1, 2
Affiliation
|
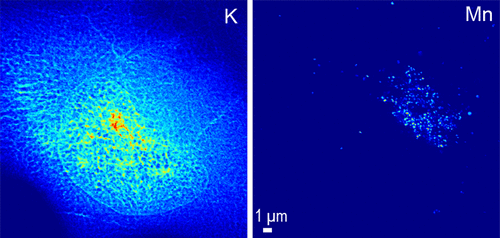
Manganese (Mn) is an essential metal that can be neurotoxic when elevated exposition occurs leading to parkinsonian-like syndromes. Mutations in the Slc30a10 gene have been identified in new forms of familial parkinsonism. SLC30A10 is a cell surface protein involved in the efflux of Mn and protects the cell against Mn toxicity. Disease-causing mutations block the efflux activity of SLC30A10, resulting in Mn accumulation. Determining the intracellular localization of Mn when disease-causing SLC30A10 mutants are expressed is essential to elucidate the mechanisms of Mn neurotoxicity. Here, using organelle fluorescence microscopy and synchrotron X-ray fluorescence (SXRF) imaging, we found that Mn accumulates in the Golgi apparatus of human cells transfected with the disease-causing SLC30A10-Δ105-107 mutant under physiological conditions and after exposure to Mn. In cells expressing the wild-type SLC30A10 protein, cellular Mn content was low after all exposure conditions, confirming efficient Mn efflux. In nontransfected cells that do not express endogenous SLC30A10 and in mock transfected cells, Mn was located in the Golgi apparatus, similarly to its distribution in cells expressing the mutant protein, confirming deficient Mn efflux. The newly developed SXRF cryogenic nanoimaging (<50 nm resolution) indicated that Mn was trapped in single vesicles within the Golgi apparatus. Our results confirm the role of SLC30A10 in Mn efflux and the accumulation of Mn in cells expressing the disease-causing SLC30A10-Δ105-107 mutation. Moreover, we identified suborganelle Golgi nanovesicles as the main compartment of Mn accumulation in SLC30A10 mutants, suggesting interactions with the vesicular trafficking machinery as a cause of the disease.
中文翻译:

SLC30A10突变与帕金森氏症有关,导致高尔基体纳米囊中锰的积累。
锰(Mn)是一种必需金属,当暴露量增加导致帕金森氏症候群时,可能具有神经毒性。Slc30a10基因的突变已在家族性帕金森症的新形式中得到确认。SLC30A10是一种参与Mn流出的细胞表面蛋白,可保护细胞免受Mn毒性。引起疾病的突变会阻止SLC30A10的外排活性,从而导致Mn积累。表达引起疾病的SLC30A10突变体时确定Mn的细胞内定位对于阐明Mn神经毒性的机制至关重要。在这里,使用细胞器荧光显微镜和同步加速器X射线荧光(SXRF)成像,我们发现,在生理条件下以及暴露于Mn后,Mn会在感染了致病SLC30A10-Δ105-107突变体的人类细胞的高尔基体中蓄积。在表达野生型SLC30A10蛋白的细胞中,在所有暴露条件下,细胞中的Mn含量均较低,从而证实了有效的Mn外排。在不表达内源性SLC30A10的未转染细胞中以及在模拟转染的细胞中,Mn位于高尔基体中,这与在突变蛋白中表达的细胞中锰的分布相似,从而证实了锰流出不足。新开发的SXRF低温纳米成像(<50 nm分辨率)表明Mn被捕获在高尔基体中的单个囊泡中。我们的结果证实了SLC30A10在Mn外流中的作用以及Mn在表达引起疾病的SLC30A10-Δ105-107突变的细胞中的积累。此外,我们确定亚细胞器高尔基体纳米囊泡是SLC30A10突变体中Mn积累的主要区室,表明与囊泡运输机制的相互作用是该病的原因。
更新日期:2018-10-01
中文翻译:

SLC30A10突变与帕金森氏症有关,导致高尔基体纳米囊中锰的积累。
锰(Mn)是一种必需金属,当暴露量增加导致帕金森氏症候群时,可能具有神经毒性。Slc30a10基因的突变已在家族性帕金森症的新形式中得到确认。SLC30A10是一种参与Mn流出的细胞表面蛋白,可保护细胞免受Mn毒性。引起疾病的突变会阻止SLC30A10的外排活性,从而导致Mn积累。表达引起疾病的SLC30A10突变体时确定Mn的细胞内定位对于阐明Mn神经毒性的机制至关重要。在这里,使用细胞器荧光显微镜和同步加速器X射线荧光(SXRF)成像,我们发现,在生理条件下以及暴露于Mn后,Mn会在感染了致病SLC30A10-Δ105-107突变体的人类细胞的高尔基体中蓄积。在表达野生型SLC30A10蛋白的细胞中,在所有暴露条件下,细胞中的Mn含量均较低,从而证实了有效的Mn外排。在不表达内源性SLC30A10的未转染细胞中以及在模拟转染的细胞中,Mn位于高尔基体中,这与在突变蛋白中表达的细胞中锰的分布相似,从而证实了锰流出不足。新开发的SXRF低温纳米成像(<50 nm分辨率)表明Mn被捕获在高尔基体中的单个囊泡中。我们的结果证实了SLC30A10在Mn外流中的作用以及Mn在表达引起疾病的SLC30A10-Δ105-107突变的细胞中的积累。此外,我们确定亚细胞器高尔基体纳米囊泡是SLC30A10突变体中Mn积累的主要区室,表明与囊泡运输机制的相互作用是该病的原因。


























 京公网安备 11010802027423号
京公网安备 11010802027423号