Nature Materials ( IF 41.2 ) Pub Date : 2018-10-01 , DOI: 10.1038/s41563-018-0165-7 Aron Walsh , Alexey A. Sokol , John Buckeridge , David O. Scanlon , C. Richard A. Catlow
|
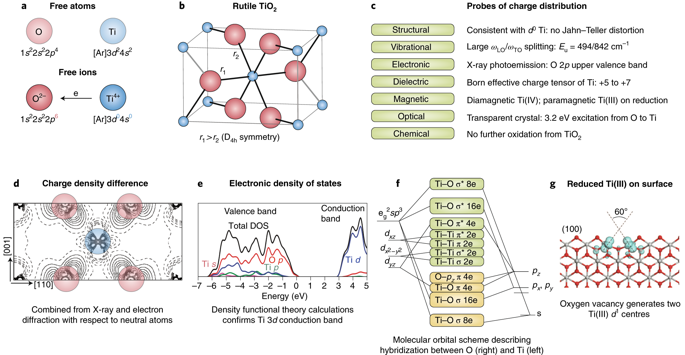
The concepts of oxidation state and atomic charge are entangled in modern materials science. We distinguish between these quantities and consider their fundamental limitations and utility for understanding material properties. We discuss the nature of bonding between atoms and the techniques that have been developed for partitioning electron density. While formal oxidation states help us count electrons (in ions, bonds, lone pairs), variously defined atomic charges are usefully employed in the description of physical processes including dielectric response and electronic spectroscopies. Such partial charges are introduced as quantitative measures in simple mechanistic models of a more complex reality, and therefore may not be comparable or transferable. In contrast, oxidation states are defined to be universal, with deviations constituting exciting challenges as evidenced in mixed-valence compounds, electrides and highly correlated systems. This Perspective covers how these concepts have evolved in recent years, our current understanding and their significance.
中文翻译:

氧化态和离子性
氧化态和原子电荷的概念在现代材料科学中纠缠不清。我们区分这些数量,并考虑它们的基本局限性和对理解材料特性的实用性。我们讨论了原子间键合的性质以及为划分电子密度而开发的技术。虽然形式上的氧化态可以帮助我们计算电子(以离子,键,孤对形式),但在定义物理过程(包括介电响应和电子光谱)时,可以有效地采用各种定义的原子电荷。这种部分费用是在更复杂的现实的简单机械模型中作为定量度量引入的,因此可能不具有可比性或可转让性。相反,氧化态定义为通用的 在混合价化合物,电子和高度相关的系统中,偏差构成了令人兴奋的挑战。本“观点”涵盖了这些概念在最近几年中的演变,我们当前的理解及其意义。


























 京公网安备 11010802027423号
京公网安备 11010802027423号