当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
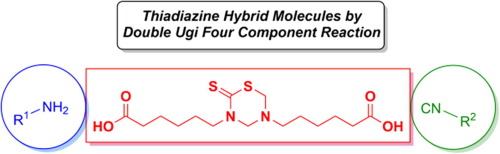
A bidirectional access to novel thiadiazine hybrid molecules by double multicomponent reactions
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-09-29 , DOI: 10.1016/j.tetlet.2018.09.070 Radell Echemendía , Waléria F. Rabêlo , Ernesto R. López , Julieta Coro , Margarita Suárez , Marcio Weber Paixão , Daniel G. Rivera
中文翻译:

通过双多组分反应双向获得新型噻二嗪杂化分子
更新日期:2018-09-29
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-09-29 , DOI: 10.1016/j.tetlet.2018.09.070 Radell Echemendía , Waléria F. Rabêlo , Ernesto R. López , Julieta Coro , Margarita Suárez , Marcio Weber Paixão , Daniel G. Rivera
|
In an attempt to further exploit multicomponent reactions in the field of hybrid heterocyclic molecules, we describe a bidirectional approach for the synthesis of novel 1,3,5-thiadiazine-peptides molecules. The process relies on the execution of two Ugi reactions between dicarboxy-functionalized 1,3,5-thiadiazine with different amines and isocyanides. An alternative strategy relying on a sequential multicomponent macrocyclization procedure was also developed to afford thiadiazine peptide-like macrocycles. Both methods showed great chemical efficiency and versatility.
中文翻译:

通过双多组分反应双向获得新型噻二嗪杂化分子
为了进一步开发杂化杂环分子领域中的多组分反应,我们描述了一种合成新型1,3,5-噻二嗪-肽分子的双向方法。该方法依赖于在二羧基官能化的1,3,5-噻二嗪与不同的胺和异氰酸酯之间进行两次Ugi反应。还开发了一种依赖于顺序多组分大环化程序的替代策略,以提供噻二嗪肽样大环化合物。两种方法均显示出很高的化学效率和多功能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号