当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantitative Modeling of the Temperature Dependence of the Kinetic Parameters for Zirconium Amine Bis(Phenolate) Catalysts for 1-Hexene Polymerization
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acscatal.8b01989 Jeffrey M. Switzer 1 , Paul D. Pletcher 2 , D. Keith Steelman 2 , Jungsuk Kim 1 , Grigori A. Medvedev 1 , Mahdi M. Abu-Omar 2 , James M. Caruthers 1 , W. Nicholas Delgass 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acscatal.8b01989 Jeffrey M. Switzer 1 , Paul D. Pletcher 2 , D. Keith Steelman 2 , Jungsuk Kim 1 , Grigori A. Medvedev 1 , Mahdi M. Abu-Omar 2 , James M. Caruthers 1 , W. Nicholas Delgass 1
Affiliation
|
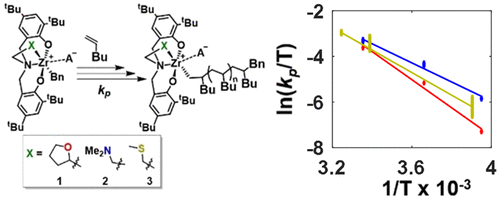
The chemical kinetics for a series of three zirconium amine bis(phenolate) catalysts for poly 1-hexene polymerization have been examined as a function of temperature. Detailed modeling of the experimental data has yielded the activation parameters for many of the reaction rate constants, including those for propagation, initiation, chain transfer, and monomer misinsertion and recovery. While existing literature is sparse, the results herein generally agree with previously published values; specifically, for the propagation rate constant, the magnitude of the enthalpy of activation is low (12 kcal mol–1 and below), and the magnitude of the entropy of activation is moderate (up to −27 cal mol–1 K–1). With regard to the remaining rate constants, Arrhenius behavior is observed in most cases despite the complexity of the temperature dependence in the two-step adsorption/insertion kinetics. The rate expression for these reactions approaches an Arrhenius form in certain limiting cases of the relative elementary rate constants. The reaction rate data are compared against these limiting cases, leading to the conclusion that docking, with an early transition state, is rate limiting for propagation and placing bounds on the interpretation of most of the remaining constants. The challenges encountered in assigning temperature-dependent rate constants for the polymerization reactions are indicative of both the significant complexity in modeling a reaction in which thousands of species are present and the extreme care that is required in generating reproducible data.
中文翻译:

1-己烯聚合锆胺双(酚酸酯)催化剂动力学参数对温度依赖性的定量建模
已经研究了用于聚1-己烯聚合的一系列三种锆胺双(酚盐)催化剂的化学动力学随温度的变化。对实验数据进行详细的建模,得出了许多反应速率常数的活化参数,包括传播,引发,链转移以及单体错误插入和回收的常数。尽管现有文献稀疏,但此处的结果通常与以前发表的值一致;具体来说,对于传播速率常数,活化焓的幅度较低(12 kcal mol –1及以下),活化熵的强度适中(最高-27 cal mol –1 K –1))。关于剩余的速率常数,尽管在两步吸附/插入动力学中温度依赖性很复杂,但在大多数情况下仍观察到阿累尼乌斯行为。在相对基本速率常数的某些限制情况下,这些反应的速率表达接近Arrhenius形式。将反应速率数据与这些极限情况进行了比较,得出的结论是,在较早的过渡状态下,对接是传播速率的限制,并限制了大多数剩余常数的解释。
更新日期:2018-09-27
中文翻译:

1-己烯聚合锆胺双(酚酸酯)催化剂动力学参数对温度依赖性的定量建模
已经研究了用于聚1-己烯聚合的一系列三种锆胺双(酚盐)催化剂的化学动力学随温度的变化。对实验数据进行详细的建模,得出了许多反应速率常数的活化参数,包括传播,引发,链转移以及单体错误插入和回收的常数。尽管现有文献稀疏,但此处的结果通常与以前发表的值一致;具体来说,对于传播速率常数,活化焓的幅度较低(12 kcal mol –1及以下),活化熵的强度适中(最高-27 cal mol –1 K –1))。关于剩余的速率常数,尽管在两步吸附/插入动力学中温度依赖性很复杂,但在大多数情况下仍观察到阿累尼乌斯行为。在相对基本速率常数的某些限制情况下,这些反应的速率表达接近Arrhenius形式。将反应速率数据与这些极限情况进行了比较,得出的结论是,在较早的过渡状态下,对接是传播速率的限制,并限制了大多数剩余常数的解释。



























 京公网安备 11010802027423号
京公网安备 11010802027423号