当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational strategies to probe CH activation in dioxo-dicopper complexes†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1039/c8cp05096a Zhenzhuo Lan 1, 2, 3, 4, 5 , Shaama Mallikarjun Sharada 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1039/c8cp05096a Zhenzhuo Lan 1, 2, 3, 4, 5 , Shaama Mallikarjun Sharada 1, 2, 3, 4, 5
Affiliation
|
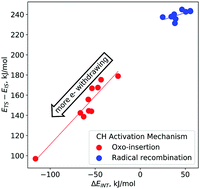
We employ density functional theory and energy decomposition analysis to probe the mechanism of CH activation in dioxo-dicopper complexes. The electrophilicity of monodentate N-donor ligands coordinated to Cu is systematically varied to examine the response of barriers to the two proposed pathways – one-step oxo-insertion and two-step radical recombination. Electron-withdrawing ligand stabilize the oxo-insertion transition state via charge transfer interactions, and therefore lead to lower barriers. On the other hand, barriers to the CH activation step in the radical recombination mechanism exhibit almost no dependence on N-donor electrophilicity. Based on the similarities between calculated and experimental Hammett relationships, the oxo-insertion pathway appears to be the preferred mechanism of CH activation in dioxo-dicopper catalysts.
中文翻译:

探测二氧杂双铜配合物中CH活化的计算策略†
我们采用密度泛函理论和能量分解分析来探讨二氧杂双铜配合物中CH活化的机理。系统地改变了与铜配位的单齿N-供体配体的亲电子性,以检查屏障对两种拟议途径的反应-一步氧代插入和两步自由基重组。吸电子配体通过电荷转移相互作用,因此导致较低的势垒。另一方面,自由基重组机制中CH活化步骤的障碍几乎不依赖于N-给体亲电性。基于计算的和实验的哈米特关系之间的相似性,在二氧代-二铜催化剂中,氧代插入途径似乎是CH活化的优选机制。
更新日期:2018-09-27
中文翻译:

探测二氧杂双铜配合物中CH活化的计算策略†
我们采用密度泛函理论和能量分解分析来探讨二氧杂双铜配合物中CH活化的机理。系统地改变了与铜配位的单齿N-供体配体的亲电子性,以检查屏障对两种拟议途径的反应-一步氧代插入和两步自由基重组。吸电子配体通过电荷转移相互作用,因此导致较低的势垒。另一方面,自由基重组机制中CH活化步骤的障碍几乎不依赖于N-给体亲电性。基于计算的和实验的哈米特关系之间的相似性,在二氧代-二铜催化剂中,氧代插入途径似乎是CH活化的优选机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号