Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2018-09-26 , DOI: 10.1016/j.jfluchem.2018.09.010 Daisuke Watanabe , Daisuke Akiyama , Nobuaki Sato
|
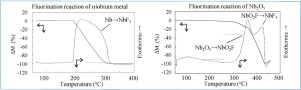
Niobium is one of the fission products contained in spent nuclear fuel. Since niobium pentafluoride (NbF5) has high volatility, it is considered that niobium volatilizes with uranium hexafluoride when applying the fluoride volatility method, which is a promising pyro-reprocessing method. In this study, fluorination behavior characteristics of niobium compounds, such as reaction temperature, volatility, and reaction path, were investigated by thermogravimetric and differential thermal analyses and X-ray diffraction analysis. The target compounds were niobium metal, the niobium oxides NbO, Nb2O3, NbO2 and Nb2O5, and niobium oxyfluoride (NbO2F). All the niobium compounds reacted exothermically and were volatilized completely by the reaction with F2. It was considered that niobium volatilized as NbF5. The fluorination reactions started respectively at 180, 200, 300, and 300 °C for niobium metal, NbO, NbO2 and Nb2O5. In the fluorination of niobium oxides, the intermediate product NbO2F was also fluorinated above 300 °C and volatilized completely. Nb2O3, which seemed to be a mixture of NbO and NbO2, reacted with F2 as described by the summation of the fluorination reactions of NbO and NbO2. The reaction mechanism for the fluorination of niobium compounds obtained in this study is applicable to evaluation of the niobium transfer phenomena in the reprocessing process of the fluoride volatility method.
中文翻译:

氟化物用于氟化铌化合物的挥发性方法
铌是乏核燃料中所包含的裂变产物之一。由于五氟化铌(NbF 5)具有高挥发性,因此在应用氟化物挥发性方法时,认为铌会与六氟化铀一起挥发,这是一种很有前途的热加工方法。在这项研究中,通过热重分析和差热分析以及X射线衍射分析研究了铌化合物的氟化行为特征,例如反应温度,挥发度和反应路径。目标化合物是铌金属,铌氧化物NbO,Nb 2 O 3,NbO 2和Nb 2 O 5以及氟化氧铌(NbO 2F)。所有的铌化合物都发生放热反应,并通过与F 2的反应而完全挥发。认为铌挥发为NbF 5。铌金属,NbO,NbO 2和Nb 2 O 5的氟化反应分别在180、200、300和300°C下开始。在氟化铌的氟化过程中,中间产物NbO 2 F也在300°C以上氟化并完全挥发。Nb 2 O 3似乎是NbO和NbO 2的混合物,它与F 2反应,如NbO和NbO 2的氟化反应总和所述。。本研究中得到的铌化合物的氟化反应机理可用于评估氟化物挥发性方法的后处理过程中的铌转移现象。


























 京公网安备 11010802027423号
京公网安备 11010802027423号