当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
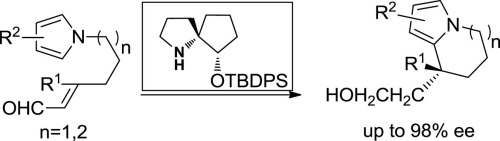
Asymmetric intramolecular Friedel–Crafts reaction catalyzed by a spiropyrrolidine organocatalyst: Enantioselective construction of indolizine and azepine frameworks
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-09-26 , DOI: 10.1016/j.tetlet.2018.09.061 Yi-Hang Zhang , Yong-Hai Yuan , Shu-Yu Zhang , Yong-Qiang Tu , Jin-Miao Tian
中文翻译:

螺吡咯烷有机催化剂催化的不对称分子内Friedel-Crafts反应:吲哚嗪和氮杂骨架的对映选择性结构
更新日期:2018-09-26
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2018-09-26 , DOI: 10.1016/j.tetlet.2018.09.061 Yi-Hang Zhang , Yong-Hai Yuan , Shu-Yu Zhang , Yong-Qiang Tu , Jin-Miao Tian
|
The asymmetric intramolecular Friedel–Crafts type Michael reaction of α,β-unsaturated aldehyde with pyrrole, catalyzed by a spiropyrrolidine (SP)-type organocatalyst, has been accomplished, which allows the construction of a series of azepine and indolizine frameworks with high to excellent enantioselectivities (up to 98% ee). Moreover, the substrate scope could be extended to generate a quaternary center in indolizine frameworks (up to 96% ee).
中文翻译:

螺吡咯烷有机催化剂催化的不对称分子内Friedel-Crafts反应:吲哚嗪和氮杂骨架的对映选择性结构
螺吡咯烷(SP)型有机催化剂催化的α,β-不饱和醛与吡咯的不对称分子内弗里德-克来福特米歇尔反应得以完成,从而可以构建一系列从高到优的氮杂环庚烷和吲哚嗪骨架对映选择性(高达98%ee)。此外,可以扩大底物的范围以在吲哚嗪框架中生成四元中心(ee高达96%)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号