当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Neighboring Component Effect in a Tristable [2]Rotaxane
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-09-25 , DOI: 10.1021/jacs.8b08519 Yuping Wang 1 , Tao Cheng 2 , Junling Sun 1 , Zhichang Liu 3 , Marco Frasconi 4 , William A. Goddard 2 , J. Fraser Stoddart 1, 5, 6
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-09-25 , DOI: 10.1021/jacs.8b08519 Yuping Wang 1 , Tao Cheng 2 , Junling Sun 1 , Zhichang Liu 3 , Marco Frasconi 4 , William A. Goddard 2 , J. Fraser Stoddart 1, 5, 6
Affiliation
|
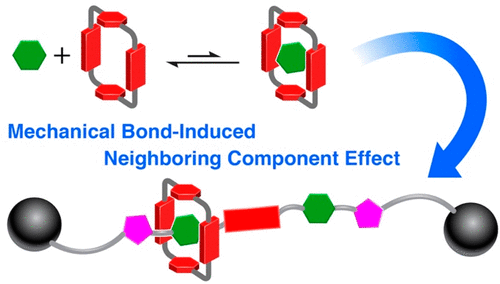
The redox properties of cyclobis(paraquat- p-phenylene)cyclophane (CBPQT4+) render it a uniquely variable source of recognition in the context of mechanically interlocked molecules, through aromatic donor-acceptor interactions in its fully oxidized state (CPBQT4+) and radical-pairing interactions in its partially reduced state (CBPQT2(•+)). Although it is expected that the fully reduced neutral state (CBPQT(0)) might behave as a π-donating recognition unit, resulting in a dramatic change in its binding properties when compared with the other two redox states, its role in rotaxanes has not yet been investigated. To address this challenge, we report herein the synthesis of a tri-stable [2]rotaxane in which a CBPQT4+ ring is mechanically interlocked with a dumbbell component containing five recognition sites-(i) a bipyridinium radical cation (BIPY(•+)) located centrally along the axis of the dumbbell, straddled by (ii) two tetrafluorophenylene units linked to (iii) two triazole rings. In addition to the selective recognition between (iv) the CBPQT4+ ring and the triazole units, and (v) the CBPQT2(•+) ring and the reduced BIPY(•+) unit in the dumbbell component, investigations in solution have now confirmed the presence of additional non-covalent bonding interactions between the CBPQT(0) ring, acting as a donor in its neutral state, and the two tetrafluorophenylene acceptors in the dumbbell component. The unveiling of this piece of molecular recognition in a [2]rotaxane is reminiscent of the existence in much simpler, covalently linked, organic molecules of neighboring group participation (anchimeric assistance giving way to transannular interactions) in small-, medium-, and large-membered rings.
中文翻译:

三稳态 [2] 轮烷中的相邻组分效应
环双(百草枯-对亚苯基)环烷 (CBPQT4+) 的氧化还原特性使其成为机械互锁分子背景下独特的可变识别来源,通过完全氧化状态下的芳香供体-受体相互作用 (CPBQT4+) 和自由基配对处于部分还原状态的相互作用 (CBPQT2(•+))。尽管预计完全还原的中性状态 (CBPQT(0)) 可能表现为 π 供体识别单元,与其他两种氧化还原状态相比,其结合性质发生了巨大变化,但其在轮烷中的作用并未尚未调查。为了应对这一挑战,我们在此报告了三稳定 [2] 轮烷的合成,其中 CBPQT4+ 环与包含五个识别位点的哑铃组件机械互锁 - (i) 位于轴中心的联吡啶自由基阳离子 (BIPY(•+))哑铃,由 (ii) 连接到 (iii) 两个三唑环的两个四氟亚苯基单元跨越。除了 (iv) CBPQT4+ 环和三唑单元之间的选择性识别,以及 (v) CBPQT2(•+) 环和哑铃组件中还原的 BIPY(•+) 单元之间的选择性识别之外,溶液中的研究现已证实CBPQT(0) 环之间存在额外的非共价键相互作用,作为中性状态的供体,与哑铃组件中的两个四氟亚苯基受体。
更新日期:2018-09-25
中文翻译:

三稳态 [2] 轮烷中的相邻组分效应
环双(百草枯-对亚苯基)环烷 (CBPQT4+) 的氧化还原特性使其成为机械互锁分子背景下独特的可变识别来源,通过完全氧化状态下的芳香供体-受体相互作用 (CPBQT4+) 和自由基配对处于部分还原状态的相互作用 (CBPQT2(•+))。尽管预计完全还原的中性状态 (CBPQT(0)) 可能表现为 π 供体识别单元,与其他两种氧化还原状态相比,其结合性质发生了巨大变化,但其在轮烷中的作用并未尚未调查。为了应对这一挑战,我们在此报告了三稳定 [2] 轮烷的合成,其中 CBPQT4+ 环与包含五个识别位点的哑铃组件机械互锁 - (i) 位于轴中心的联吡啶自由基阳离子 (BIPY(•+))哑铃,由 (ii) 连接到 (iii) 两个三唑环的两个四氟亚苯基单元跨越。除了 (iv) CBPQT4+ 环和三唑单元之间的选择性识别,以及 (v) CBPQT2(•+) 环和哑铃组件中还原的 BIPY(•+) 单元之间的选择性识别之外,溶液中的研究现已证实CBPQT(0) 环之间存在额外的非共价键相互作用,作为中性状态的供体,与哑铃组件中的两个四氟亚苯基受体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号