当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational Discovery of Li–M–O Ion Exchange Materials for Lithium Extraction from Brines
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-09-25 00:00:00 , DOI: 10.1021/acs.chemmater.7b03509 David H. Snydacker 1 , Vinay I. Hegde 1 , Muratahan Aykol 1 , C. Wolverton 1
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-09-25 00:00:00 , DOI: 10.1021/acs.chemmater.7b03509 David H. Snydacker 1 , Vinay I. Hegde 1 , Muratahan Aykol 1 , C. Wolverton 1
Affiliation
|
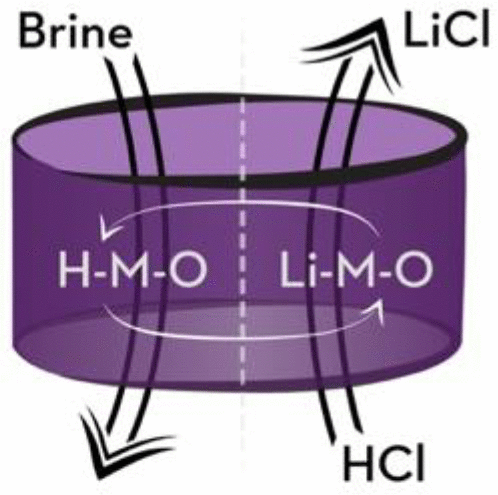
Lithium is an essential element for today’s high-performance batteries. Brine resources contain most of the world’s lithium reserves, but conventional processes for extracting lithium from brines are limited by low lithium recovery and large evaporation ponds. Lithium ion exchange is an alternative extraction method with potential to access lower-quality resources and decrease costs. Ion exchange materials absorb lithium from brine resources and then release the lithium in acid while absorbing hydrogen. New ion exchange materials are sought to facilitate this transformative approach. We use high-throughput density functional theory and specific ion interaction theory to predict promising new lithium metal oxide compounds suitable for lithium extraction. Starting from the Open Quantum Materials Database (OQMD) of ∼400,000 compounds, we consider 77 candidate lithium metal oxide compounds that are stable or nearly stable in their lithiated states. We interrogate this list for compounds that thermodynamically release lithium while binding hydrogen in acid and that also release hydrogen while binding lithium in brine. We further screen for selective binding of lithium relative to sodium in brine. We find that most candidate compounds either bind lithium in both acid and brine solutions or bind hydrogen in both acid and brine solutions. Such compounds are not suitable for lithium ion exchange. However, we identify nine compounds that are most promising for lithium extraction from brines: LiAlO2, LiCuO2, Li2MnO3, Li4Mn5O12, Li2SnO3, Li4TiO4, Li4Ti5O12, Li7Ti11O24, and Li3VO4. Four additional compounds are promising when the pH of the brine is adjusted to 10 to help drive hydrogen release: Li2TiO3, LiTiO2, Li2FeO3, and Li2Si3O7. Four of the previously mentioned compounds are also promising for Li extraction from seawater: Li2MnO3, Li4Mn5O12, Li7Ti11O24, and Li3VO4.
中文翻译:

从盐水中提取锂的Li–M–O离子交换材料的计算发现
锂是当今高性能电池必不可少的元素。盐水资源包含世界上大部分的锂储量,但是常规的从盐水中提取锂的方法受到锂回收率低和蒸发池大的限制。锂离子交换是一种替代性提取方法,具有获取较低质量资源并降低成本的潜力。离子交换材料从盐水资源中吸收锂,然后在酸中释放锂,同时吸收氢。寻求新的离子交换材料以促进这种转化方法。我们使用高通量密度泛函理论和比离子相互作用理论来预测有前途的新型锂金属氧化物化合物,适用于锂提取。从约40万种化合物的开放量子材料数据库(OQMD)开始,我们考虑了77种在锂化状态下稳定或接近稳定的锂金属氧化物化合物。我们查询了该列表,列出了在酸中与氢结合时热力学释放锂,而在盐水中与锂结合时也释放氢的化合物。我们进一步筛选了锂相对于盐水中钠的选择性结合。我们发现大多数候选化合物要么在酸和盐溶液中都结合锂,要么在酸和盐溶液中都结合氢。这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO 我们查询了该列表,列出了在酸与氢结合时热力学释放锂,在盐水与锂结合时释放氢的化合物。我们进一步筛选了锂相对于盐水中钠的选择性结合。我们发现大多数候选化合物要么在酸和盐溶液中都结合锂,要么在酸和盐溶液中都结合氢。这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO 我们查询了该列表,列出了在酸中与氢结合时热力学释放锂,而在盐水中与锂结合时也释放氢的化合物。我们进一步筛选了锂相对于盐水中钠的选择性结合。我们发现大多数候选化合物要么在酸和盐溶液中都结合锂,要么在酸和盐溶液中都结合氢。这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO 这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO 这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO2,LiCuO 2,Li 2 MnO 3,Li 4 Mn 5 O 12,Li 2 SnO 3,Li 4 TiO 4,Li 4 Ti 5 O 12,Li 7 Ti 11 O 24和Li 3 VO 4。当将盐水的pH值调整为10以帮助推动氢释放时,有四种其他化合物很有希望:Li 2 TiO 3,LiTiO 2,Li 2 FeO 3和Li2 Si 3 O 7。前面提到的四种化合物也有望用于从海水中提取锂:Li 2 MnO 3,Li 4 Mn 5 O 12,Li 7 Ti 11 O 24和Li 3 VO 4。
更新日期:2018-09-25
中文翻译:

从盐水中提取锂的Li–M–O离子交换材料的计算发现
锂是当今高性能电池必不可少的元素。盐水资源包含世界上大部分的锂储量,但是常规的从盐水中提取锂的方法受到锂回收率低和蒸发池大的限制。锂离子交换是一种替代性提取方法,具有获取较低质量资源并降低成本的潜力。离子交换材料从盐水资源中吸收锂,然后在酸中释放锂,同时吸收氢。寻求新的离子交换材料以促进这种转化方法。我们使用高通量密度泛函理论和比离子相互作用理论来预测有前途的新型锂金属氧化物化合物,适用于锂提取。从约40万种化合物的开放量子材料数据库(OQMD)开始,我们考虑了77种在锂化状态下稳定或接近稳定的锂金属氧化物化合物。我们查询了该列表,列出了在酸中与氢结合时热力学释放锂,而在盐水中与锂结合时也释放氢的化合物。我们进一步筛选了锂相对于盐水中钠的选择性结合。我们发现大多数候选化合物要么在酸和盐溶液中都结合锂,要么在酸和盐溶液中都结合氢。这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO 我们查询了该列表,列出了在酸与氢结合时热力学释放锂,在盐水与锂结合时释放氢的化合物。我们进一步筛选了锂相对于盐水中钠的选择性结合。我们发现大多数候选化合物要么在酸和盐溶液中都结合锂,要么在酸和盐溶液中都结合氢。这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO 我们查询了该列表,列出了在酸中与氢结合时热力学释放锂,而在盐水中与锂结合时也释放氢的化合物。我们进一步筛选了锂相对于盐水中钠的选择性结合。我们发现大多数候选化合物要么在酸和盐溶液中都结合锂,要么在酸和盐溶液中都结合氢。这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO 这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO 这样的化合物不适合锂离子交换。但是,我们确定了从盐水中提取锂最有希望的九种化合物:LiAlO2,LiCuO 2,Li 2 MnO 3,Li 4 Mn 5 O 12,Li 2 SnO 3,Li 4 TiO 4,Li 4 Ti 5 O 12,Li 7 Ti 11 O 24和Li 3 VO 4。当将盐水的pH值调整为10以帮助推动氢释放时,有四种其他化合物很有希望:Li 2 TiO 3,LiTiO 2,Li 2 FeO 3和Li2 Si 3 O 7。前面提到的四种化合物也有望用于从海水中提取锂:Li 2 MnO 3,Li 4 Mn 5 O 12,Li 7 Ti 11 O 24和Li 3 VO 4。

























 京公网安备 11010802027423号
京公网安备 11010802027423号