Drug Discovery Today ( IF 7.4 ) Pub Date : 2018-09-26 , DOI: 10.1016/j.drudis.2018.09.015 Eline van Overbeeke , Chiara Whichello , Rosanne Janssens , Jorien Veldwijk , Irina Cleemput , Steven Simoens , Juhaeri Juhaeri , Bennett Levitan , Jürgen Kübler , Esther de Bekker-Grob , Isabelle Huys
|
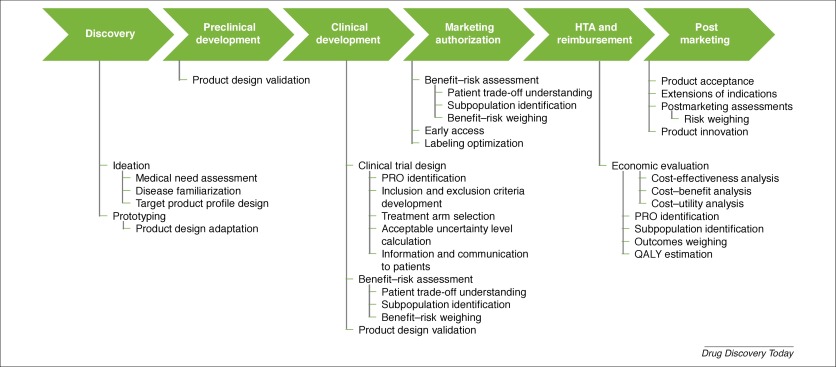
Industry, regulators, health technology assessment (HTA) bodies, and payers are exploring the use of patient preferences in their decision-making processes. In general, experience in conducting and assessing patient preference studies is limited. Here, we performed a systematic literature search and review to identify factors and situations influencing the value of patient preference studies, as well as applications throughout the medical product lifecyle. Factors and situations identified in 113 publications related to the organization, design, and conduct of studies, and to communication and use of results. Although current use of patient preferences is limited, we identified possible applications in discovery, clinical development, marketing authorization, HTA, and postmarketing phases.
中文翻译:

在医疗产品生命周期中影响患者偏好研究价值的因素和情况:文献综述
行业,监管机构,卫生技术评估(HTA)机构和付款人正在探索在决策过程中使用患者偏好的方法。通常,进行和评估患者偏好研究的经验是有限的。在这里,我们进行了系统的文献搜索和审查,以找出影响患者偏爱研究价值的因素和情况,以及在整个医疗产品生命周期中的应用。在113个出版物中确定的因素和情况与研究的组织,设计和进行以及结果的传达和使用有关。尽管目前对患者偏好的使用受到限制,但我们确定了在发现,临床开发,市场授权,HTA和上市后阶段的可能应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号