当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An alternative route for the synthesis of hydroxylated pillar[5]arene-based amphiphiles
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-26 , DOI: 10.1039/c8ob02074d Talal F. Al-Azemi 1, 2, 3, 4 , Mickey Vinodh 1, 2, 3, 4 , Fatemeh H. Alipour 1, 2, 3, 4 , Abdirahman A. Mohamod 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-26 , DOI: 10.1039/c8ob02074d Talal F. Al-Azemi 1, 2, 3, 4 , Mickey Vinodh 1, 2, 3, 4 , Fatemeh H. Alipour 1, 2, 3, 4 , Abdirahman A. Mohamod 1, 2, 3, 4
Affiliation
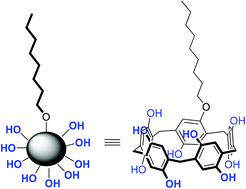
|
Conformational mobilities of the units and host–guest complexation with n-octyltrimethylammonium hexafluorophosphate of the synthesized perbenzylated pillar[5]arenes were studied. The formed complex was confirmed by proton nuclear magnetic resonance spectroscopy and mass spectral analysis. Hydroxylated pillar[5]arene-based amphiphiles were synthesized by a co-cyclization strategy followed by catalytic hydrogenation. This approach unlocks the synthesis and the design of a wide range of structural manipulations to these amphiphilic pillararenes.
中文翻译:

合成羟基化的基于柱[5]芳烃的两亲物的替代途径
研究了合成苄基柱[5]芳烃的单元构象迁移率和主客体与六氟磷酸正辛基三甲基铵的络合。通过质子核磁共振波谱和质谱分析确认了形成的络合物。通过共环化策略然后催化加氢合成羟基化的基于柱[5]芳烃的两亲物。这种方法解锁了对这些两亲性柱芳烃的各种结构操作的合成和设计。
更新日期:2018-10-18
中文翻译:

合成羟基化的基于柱[5]芳烃的两亲物的替代途径
研究了合成苄基柱[5]芳烃的单元构象迁移率和主客体与六氟磷酸正辛基三甲基铵的络合。通过质子核磁共振波谱和质谱分析确认了形成的络合物。通过共环化策略然后催化加氢合成羟基化的基于柱[5]芳烃的两亲物。这种方法解锁了对这些两亲性柱芳烃的各种结构操作的合成和设计。


























 京公网安备 11010802027423号
京公网安备 11010802027423号