当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed heck-type cascade cyclization of (Z)-1-iodo-1,6-dienes with N-tosyl hydrazones
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-25 , DOI: 10.1039/c8ob01821a Jun Li 1, 2, 3, 4 , Xiaochen Chi 1, 2, 3, 4 , Long Meng 1, 2, 3, 4 , Luyang Jiao 1, 2, 3, 4 , Wenhui Shang 1, 2, 3, 4 , Ping Wang 1, 2, 3, 4 , Daopeng Zhang 1, 2, 3, 4 , Yunhui Dong 1, 2, 3, 4 , Qing Liu 1, 2, 3, 4 , Hui Liu 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-25 , DOI: 10.1039/c8ob01821a Jun Li 1, 2, 3, 4 , Xiaochen Chi 1, 2, 3, 4 , Long Meng 1, 2, 3, 4 , Luyang Jiao 1, 2, 3, 4 , Wenhui Shang 1, 2, 3, 4 , Ping Wang 1, 2, 3, 4 , Daopeng Zhang 1, 2, 3, 4 , Yunhui Dong 1, 2, 3, 4 , Qing Liu 1, 2, 3, 4 , Hui Liu 1, 2, 3, 4
Affiliation
|
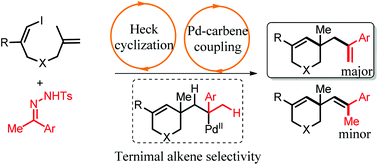
A palladium-catalyzed heck-type cascade cyclization of (Z)-1-iodo-1,6-dienes with N-tosyl hydrazones is reported. The alkylpalladium intermediate coupled with the diazo compound, generating the second alkylpalladium species bearing two β-H, which generated a terminal alkene as the major products in the anti-Zaitsev way via the highly regioselective β-H elimination. It provided a new way to synthesize tetrahydropyridine derivatives bearing a terminal alkene.
中文翻译:

钯催化的N-甲苯磺酰基的(Z)-1-碘-1,6-二烯的钯催化的heck型级联环化
报道了钯催化的N-甲苯磺酰基对(Z)-1-碘-1,6-二烯的钯催化的heck型级联环化。烷基钯中间体与重氮化合物偶联,生成带有两个β-H的第二个烷基钯物种,后者通过高度区域选择性的β-H消除,以反Zaitsev方式生成末端烯烃作为主要产物。它提供了一种合成带有末端烯烃的四氢吡啶衍生物的新方法。
更新日期:2018-10-18
中文翻译:

钯催化的N-甲苯磺酰基的(Z)-1-碘-1,6-二烯的钯催化的heck型级联环化
报道了钯催化的N-甲苯磺酰基对(Z)-1-碘-1,6-二烯的钯催化的heck型级联环化。烷基钯中间体与重氮化合物偶联,生成带有两个β-H的第二个烷基钯物种,后者通过高度区域选择性的β-H消除,以反Zaitsev方式生成末端烯烃作为主要产物。它提供了一种合成带有末端烯烃的四氢吡啶衍生物的新方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号