当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Blood exosomes regulate the tissue distribution of grapefruit-derived nanovector via CD36 and IGFR1 pathways.
Theranostics ( IF 12.4 ) Pub Date : 2018-09-09 , DOI: 10.7150/thno.27608 Qi-Long Wang 1 , XiaoYing Zhuang 2 , Mukesh K Sriwastva 2 , Jingyao Mu 2 , Yun Teng 2 , Zhongbin Deng 2 , Lifeng Zhang 2 , Kumaran Sundaram 2 , Anil Kumar 2 , Donald Miller 2 , Jun Yan 2, 3 , Huang-Ge Zhang 2, 4
Theranostics ( IF 12.4 ) Pub Date : 2018-09-09 , DOI: 10.7150/thno.27608 Qi-Long Wang 1 , XiaoYing Zhuang 2 , Mukesh K Sriwastva 2 , Jingyao Mu 2 , Yun Teng 2 , Zhongbin Deng 2 , Lifeng Zhang 2 , Kumaran Sundaram 2 , Anil Kumar 2 , Donald Miller 2 , Jun Yan 2, 3 , Huang-Ge Zhang 2, 4
Affiliation
|
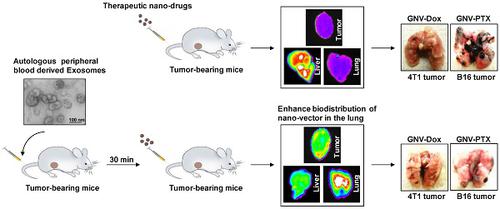
Tumor-specific delivery of therapeutics is challenging. One of the major hurdles for successfully delivering targeted agents by nanovectors is the filtering role of the liver in rapidly sequestering nanovectors from the circulation. Exosomes, a type of endogenous nanoparticle, circulate continuously in the peripheral blood and play a role in intercellular communication. The aim of this study was to determine whether the level of endogenous exosomes has an effect on nanovector delivery efficiency of targeted agents. Methods: Exosomes were isolated from peripheral blood and intravenously (I.V.) injected into tumor-bearing mice. Subsequently, 1,1-dioctadecyl-3,3,3'3'-tetramethylindotricarbocyanine-iodide (DiR) fluorescent dye-labeled nanoparticles, including grapefruit nanovectors (GNV) and standard liposomes, were I.V. injected in the mice. The efficiency of redirecting GNVs from liver to other organs of injected mice was further analyzed with in vivo imaging. The concentration of chemo drugs delivered by GNV was measured by HPLC and the anti-lung metastasis therapeutic effects of chemo drugs delivered by GNVs in mouse breast cancer and melanoma cancer models were evaluated. Results: We show that tail vein-injected exosomes isolated from mouse peripheral blood were predominately taken up by liver Kupffer cells. Injection of peripheral blood-derived exosomes before I.V. injection of grapefruit-derived nanovector (GNV) decreased the deposition of GNV in the liver and redirected the GNV to the lung and to the tumor in breast and melanoma tumor-bearing mouse models. Enhanced therapeutic efficiency of doxorubicin (Dox) or paclitaxel (PTX) carried by GNVs for lung metastases was demonstrated when there was an I.V. injection of exosomes before therapeutic treatment. Furthermore, we found that CD36 and IGFR1 receptor-mediated pathways played a critical role in the exosome-mediated inhibitory effect of GNV entry into liver macrophages. Conclusions: Collectively, our findings provide a foundation for using autologous exosomes to enhance therapeutic vector targeted delivery to the lung.
中文翻译:

血液外来体通过CD36和IGFR1途径调节葡萄柚衍生的纳米载体的组织分布。
治疗剂的肿瘤特异性递送是具有挑战性的。通过纳米载体成功递送靶向药物的主要障碍之一是肝脏在从循环中快速隔离纳米载体的过滤作用。外来体是一种内源性纳米粒子,在外周血中连续循环并在细胞间通讯中发挥作用。这项研究的目的是确定内源性外来体的水平是否对靶向药物的纳米载体传递效率有影响。方法:从外周血中分离外泌体,然后静脉注射(IV)到荷瘤小鼠体内。随后,将1,1-二十八烷基-3,3,3'3'-四甲基吲哚并花青碘(DiR)荧光染料标记的纳米颗粒,包括葡萄柚纳米载体(GNV)和标准脂质体,静脉注射到小鼠体内。通过体内成像进一步分析了将GNV从肝脏重定向到注射小鼠的其他器官的效率。通过HPLC测量由GNV递送的化学药物的浓度,并且评估由GNV递送的化学药物在小鼠乳腺癌和黑素瘤癌症模型中的抗肺转移治疗作用。结果:我们显示,从小鼠外周血中分离出的尾静脉注射型外来体主要被肝Kupffer细胞吸收。在静脉内注射葡萄柚衍生的纳米载体(GNV)之前注射外周血来源的外来体可减少GNV在肝脏中的沉积,并将GNV重定向至肺部以及荷乳腺和黑色素瘤荷瘤小鼠模型中的肿瘤。当在治疗前静脉注射外泌体时,证明了GNV携带的阿霉素(Dox)或紫杉醇(PTX)对肺转移的治疗效率得到了提高。此外,我们发现CD36和IGFR1受体介导的途径在GNV进入肝巨噬细胞的外来体介导的抑制作用中起着关键作用。结论:总的来说,我们的发现为使用自体外泌体增强治疗性载体靶向肺的递送提供了基础。
更新日期:2018-09-25
中文翻译:

血液外来体通过CD36和IGFR1途径调节葡萄柚衍生的纳米载体的组织分布。
治疗剂的肿瘤特异性递送是具有挑战性的。通过纳米载体成功递送靶向药物的主要障碍之一是肝脏在从循环中快速隔离纳米载体的过滤作用。外来体是一种内源性纳米粒子,在外周血中连续循环并在细胞间通讯中发挥作用。这项研究的目的是确定内源性外来体的水平是否对靶向药物的纳米载体传递效率有影响。方法:从外周血中分离外泌体,然后静脉注射(IV)到荷瘤小鼠体内。随后,将1,1-二十八烷基-3,3,3'3'-四甲基吲哚并花青碘(DiR)荧光染料标记的纳米颗粒,包括葡萄柚纳米载体(GNV)和标准脂质体,静脉注射到小鼠体内。通过体内成像进一步分析了将GNV从肝脏重定向到注射小鼠的其他器官的效率。通过HPLC测量由GNV递送的化学药物的浓度,并且评估由GNV递送的化学药物在小鼠乳腺癌和黑素瘤癌症模型中的抗肺转移治疗作用。结果:我们显示,从小鼠外周血中分离出的尾静脉注射型外来体主要被肝Kupffer细胞吸收。在静脉内注射葡萄柚衍生的纳米载体(GNV)之前注射外周血来源的外来体可减少GNV在肝脏中的沉积,并将GNV重定向至肺部以及荷乳腺和黑色素瘤荷瘤小鼠模型中的肿瘤。当在治疗前静脉注射外泌体时,证明了GNV携带的阿霉素(Dox)或紫杉醇(PTX)对肺转移的治疗效率得到了提高。此外,我们发现CD36和IGFR1受体介导的途径在GNV进入肝巨噬细胞的外来体介导的抑制作用中起着关键作用。结论:总的来说,我们的发现为使用自体外泌体增强治疗性载体靶向肺的递送提供了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号