Theranostics ( IF 12.4 ) Pub Date : 2018-09-09 , DOI: 10.7150/thno.27581 Xi He , Jinxiao Zhang , Chao Li , Yu Zhang , Yifei Lu , Yujie Zhang , Lisha Liu , Chunhui Ruan , Qinjun Chen , Xinli Chen , Qin Guo , Tao Sun , Jianjun Cheng , Chen Jiang
|
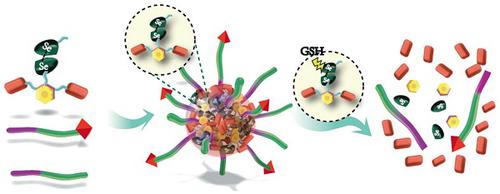
Efficient drug accumulation in tumor is essential for chemotherapy. We developed redox-responsive diselenide-based high-loading prodrug nanoparticles (NPs) for targeted triple negative breast cancer (TNBC) treatment.
Method: Redox-responsive diselenide bond (Se-Se) containing dimeric prodrug (PTXD-Se) was synthesized and co-precipitated with TNBC-targeting amphiphilic copolymers to form ultra-stable NPs (uPA-PTXD NPs). The drug loading capacity and redox-responsive drug release behavior were studied. TNBC targeting effect and anti-tumor effect were also evaluated in vitro and in vivo.
Results: On-demand designed paclitaxel dimeric prodrug could co-precipitate with amphiphilic copolymers to form ultra-stable uPA-PTXD NPs with high drug loading capacity. Diselenide bond (Se-Se) in uPA-PTXD NPs could be selectively cleaved by abnormally high reduced potential in tumor microenvironment, releasing prototype drug, thus contributing to improved anti-cancer efficacy. Endowed with TNBC-targeting ligand uPA peptide, uPA-PTXD NPs exhibited reduced systemic toxicity and enhanced drug accumulation in TNBC lesions, thus showed significant anti-tumor efficacy both in vitro and in vivo.
Conclusion: The comprehensive advantage of high drug loading, redox-controlled drug release and targeted tumor accumulation suggests uPA-PTXD NPs as a highly promising strategy for effective TNBC treatment.
Keywords: diselenide bond, nanoparticles, prodrugs, redox responsive, triple negative breast cancer
中文翻译:

增强的基于生物还原反应的基于二硒化物的二聚体前药纳米颗粒,用于三阴性乳腺癌治疗
肿瘤中有效的药物蓄积对于化疗至关重要。我们开发了基于氧化还原反应的基于二硒化物的高负荷前药纳米颗粒(NPs),用于靶向三阴性乳腺癌(TNBC)的治疗。
方法:合成含有二聚体前药(PTXD-Se)的氧化还原反应性二硒键(Se-Se),并与靶向TNBC的两亲共聚物共沉淀,以形成超稳定NP(uPA-PTXD NP)。研究了载药量和氧化还原反应性药物释放行为。TNBC的靶向作用和抗肿瘤作用也在体外和体内进行了评估。
结果:按需设计的紫杉醇二聚体前药可与两亲共聚物共沉淀,形成载药量高的超稳定uPA-PTXD NP。uPA-PTXD NP中的二硒键(Se-Se)可以被肿瘤微环境中异常高的还原电位选择性地裂解,从而释放出原型药物,从而有助于提高抗癌功效。与TNBC-靶向配体的uPA肽赋予,UPA-PTXD的NP出降低的全身性毒性和增强的药物蓄积在TNBC病变,从而既表现出显著抗肿瘤功效在体外和体内。
结论:高载药量,氧化还原控制的药物释放和靶向的肿瘤蓄积的综合优势表明,uPA-PTXD NPs是有效治疗TNBC的极有希望的策略。
关键词:二硒键,纳米粒子,前药,氧化还原反应,三阴性乳腺癌



























 京公网安备 11010802027423号
京公网安备 11010802027423号