当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preparation of polysubstituted dihydrofurans through a PhI(OAc)2-promoted haloenolcyclization of olefinic dicarbonyl compounds
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-24 , DOI: 10.1039/c8ob02161a Ji Liu 1, 2, 3, 4 , Qing-Yun Liu 1, 2, 3, 4 , Xing-Xiao Fang 1, 2, 3, 4 , Gong-Qing Liu 1, 2, 3, 4 , Yong Ling 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-24 , DOI: 10.1039/c8ob02161a Ji Liu 1, 2, 3, 4 , Qing-Yun Liu 1, 2, 3, 4 , Xing-Xiao Fang 1, 2, 3, 4 , Gong-Qing Liu 1, 2, 3, 4 , Yong Ling 1, 2, 3, 4
Affiliation
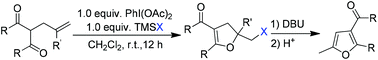
|
A metal-free cyclization of olefinic dicarbonyl compounds for the synthesis of various 5-halomethyl-4,5-dihydrofurans is presented. Using (diacetoxyiodo)benzene as the reaction promoter and halotrimethylsilane as the halogen source, the intramolecular haloenolcyclization of the 2-allyl-1,3-dicarbonyl compounds smoothly proceeded, leading to the corresponding 5-halomethyl-4,5-dihydrofurans in good to excellent isolated yields. Moreover, the resulting 5-iodomethyl products could be converted to functionalized furans in almost quantitative yields by treatment with DBU followed by acid-catalyzed rearrangement. The reactions could be carried out on a gram scale and did not require harsh reaction conditions. The good isolated yields, mild conditions, and operational simplicity make this reaction a viable method for the construction of different dihydrofuran and furan structures.
中文翻译:

通过PhI(OAc)2促进烯烃二羰基化合物的卤烯醇环化制备多取代的二氢呋喃
提出了用于合成各种5-卤代甲基-4,5-二氢呋喃的烯烃二羰基化合物的无金属环化。使用(二乙酰氧基碘)苯作为反应促进剂和卤代三甲基硅烷作为卤素源,2-烯丙基-1,3-二羰基化合物的分子内卤代烯醇环化反应顺利进行,得到了相应的5-卤代甲基-4,5-二氢呋喃。优良的孤立产量。此外,通过用DBU处理然后酸催化的重排,可以以几乎定量的产率将所得的5-碘甲基产物转化为官能化的呋喃。反应可以以克为单位进行,并且不需要苛刻的反应条件。良好的单产,温和的条件,
更新日期:2018-10-18
中文翻译:

通过PhI(OAc)2促进烯烃二羰基化合物的卤烯醇环化制备多取代的二氢呋喃
提出了用于合成各种5-卤代甲基-4,5-二氢呋喃的烯烃二羰基化合物的无金属环化。使用(二乙酰氧基碘)苯作为反应促进剂和卤代三甲基硅烷作为卤素源,2-烯丙基-1,3-二羰基化合物的分子内卤代烯醇环化反应顺利进行,得到了相应的5-卤代甲基-4,5-二氢呋喃。优良的孤立产量。此外,通过用DBU处理然后酸催化的重排,可以以几乎定量的产率将所得的5-碘甲基产物转化为官能化的呋喃。反应可以以克为单位进行,并且不需要苛刻的反应条件。良好的单产,温和的条件,



























 京公网安备 11010802027423号
京公网安备 11010802027423号