当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Optimizing Lithium Ion Conduction through Crown Ether-Based Cylindrical Channels in [Ni(dmit)2]− Salts
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1021/acs.chemmater.8b03027 Katsuya Ichihashi , Daisuke Konno , Takuya Date , Takumi Nishimura , Kseniya Yu. Maryunina , Katsuya Inoue , Toshimi Nakaya 1 , Kazuhiro Toyoda 2 , Yoko Tatewaki 3 , Tomoyuki Akutagawa 4 , Takayoshi Nakamura 5 , Sadafumi Nishihara
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-09-24 00:00:00 , DOI: 10.1021/acs.chemmater.8b03027 Katsuya Ichihashi , Daisuke Konno , Takuya Date , Takumi Nishimura , Kseniya Yu. Maryunina , Katsuya Inoue , Toshimi Nakaya 1 , Kazuhiro Toyoda 2 , Yoko Tatewaki 3 , Tomoyuki Akutagawa 4 , Takayoshi Nakamura 5 , Sadafumi Nishihara
Affiliation
|
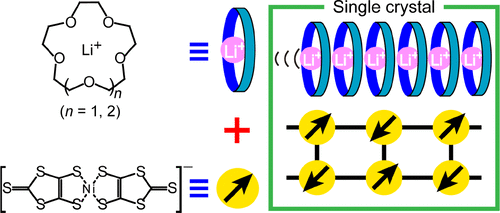
The synthesis of artificial ion channels is one of the core areas of biomimetics and is aimed at achieving control over channel functionality by careful design and selection of the constituent components. However, the optimization of ionic conductivity in the channel in the crystalline state is challenging because of crystal strain, polymorphism, and potentially limited stability. In this study, the pore size of cylindrical channels was controlled with the aim of optimizing ionic conductivity. We prepared two isomorphic salts, Li2([18]crown-6)3[Ni(dmit)2]2(H2O)4 (1) and Li2([15]crown-5)3[Ni(dmit)2]2(H2O)2 (2), both of which possess ion channels formed by a one-dimensional array of crown ethers, Li+ ions, and crystalline water molecules. Meanwhile, [Ni(dmit)2]− (S = 1/2) molecules formed a ladder configuration with Jrung/kB = −631(5) K, Jleg/kB = −185(5) K for 1, and Jrung/kB = −517(4) K, Jleg/kB = −109(5) K for 2. For 1, the Li+ ionic conductivity at 293 K in the crystalline state was enhanced from 1.89(18) × 10–8 S·cm–1 to 2.46(6) × 10–7 S·cm–1 via dehydration. Furthermore, analysis of Li+ ionic conductivities of 2, which incorporated a crown ether with a smaller cavity (the cavity diameters of [18]crown-6 and [15]crown-5 are 2.60–3.20 Å and 1.70–2.20 Å, respectively(1)) at the same temperature both before and after dehydration revealed conductivities of 1.93(31) × 10–8 S·cm–1 and 7.01(21) × 10–7 S·cm–1, respectively. This molecular design approach can contribute to increasing the ionic conductivity as well as the development of all-solid-state lithium ion batteries and other electronic device fabrications.
中文翻译:

通过[Ni(dmit)2 ] -盐中基于冠醚的圆柱通道优化锂离子传导
人工离子通道的合成是仿生技术的核心领域之一,旨在通过精心设计和选择组成成分来实现对通道功能的控制。然而,由于晶体应变,多态性和潜在的有限的稳定性,在结晶状态下通道中离子电导率的优化是具有挑战性的。在这项研究中,以优化离子电导率为目标来控制圆柱形通道的孔径。我们准备了两种同构盐,Li 2([18] cro-6)3 [Ni(dmit)2 ] 2(H 2 O)4(1)和Li 2([15] cro- 5-5 )3 [Ni(dmit) )2 ] 2(H 2 O)2(2),两者都具有由冠醚,Li +离子和结晶水分子的一维阵列形成的离子通道。同时,[Ni(dmit)2 ] -(S = 1/2)分子形成梯形,J梯级/ k B = -631(5)K,J腿/ k B = -185(5)K 1,并且J梯级/ k B = −517(4)K,J腿/ kB = -109(5)K对于2。对于1,通过脱水,在293 K晶体状态下的Li +离子电导率从1.89(18)×10 –8 S·cm –1提高到2.46(6)×10 –7 S·cm –1。此外,对Li +离子电导率2的分析,其中掺入了具有较小腔体的冠醚([18] crown-6和[15] crown-5的腔体直径分别为2.60-3.20Å和1.70-2.20Å (1))在脱水之前和之后在相同温度下的电导率分别为1.93(31)×10 –8 S·cm –1和7.01(21)×10 –7 S·cm分别为–1。这种分子设计方法可以有助于提高离子电导率,以及开发全固态锂离子电池和其他电子设备。
更新日期:2018-09-24
中文翻译:

通过[Ni(dmit)2 ] -盐中基于冠醚的圆柱通道优化锂离子传导
人工离子通道的合成是仿生技术的核心领域之一,旨在通过精心设计和选择组成成分来实现对通道功能的控制。然而,由于晶体应变,多态性和潜在的有限的稳定性,在结晶状态下通道中离子电导率的优化是具有挑战性的。在这项研究中,以优化离子电导率为目标来控制圆柱形通道的孔径。我们准备了两种同构盐,Li 2([18] cro-6)3 [Ni(dmit)2 ] 2(H 2 O)4(1)和Li 2([15] cro- 5-5 )3 [Ni(dmit) )2 ] 2(H 2 O)2(2),两者都具有由冠醚,Li +离子和结晶水分子的一维阵列形成的离子通道。同时,[Ni(dmit)2 ] -(S = 1/2)分子形成梯形,J梯级/ k B = -631(5)K,J腿/ k B = -185(5)K 1,并且J梯级/ k B = −517(4)K,J腿/ kB = -109(5)K对于2。对于1,通过脱水,在293 K晶体状态下的Li +离子电导率从1.89(18)×10 –8 S·cm –1提高到2.46(6)×10 –7 S·cm –1。此外,对Li +离子电导率2的分析,其中掺入了具有较小腔体的冠醚([18] crown-6和[15] crown-5的腔体直径分别为2.60-3.20Å和1.70-2.20Å (1))在脱水之前和之后在相同温度下的电导率分别为1.93(31)×10 –8 S·cm –1和7.01(21)×10 –7 S·cm分别为–1。这种分子设计方法可以有助于提高离子电导率,以及开发全固态锂离子电池和其他电子设备。


























 京公网安备 11010802027423号
京公网安备 11010802027423号