PLOS ONE ( IF 3.7 ) Pub Date : 2018-09-21 , DOI: 10.1371/journal.pone.0204532 Diego Sbrissa 1 , Ghassan Naisan 1 , Ognian C Ikonomov 1 , Assia Shisheva 1
|
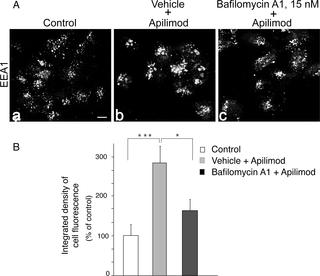
PIKfyve, an evolutionarily conserved kinase synthesizing PtdIns5P and PtdIns(3,5)P2, is crucial for mammalian cell proliferation and viability. Accordingly, PIKfyve inhibitors are now in clinical trials as anti-cancer drugs. Among those, apilimod is the most promising, yet its potency to inhibit PIKfyve and affect endomembrane homeostasis is only partially characterized. We demonstrate here for the first time that apilimod powerfully inhibited in vitro synthesis of PtdIns5P along with that of PtdIns(3,5)P2. HPLC-based resolution of intracellular phosphoinositides (PIs) revealed that apilimod triggered a marked reduction of both lipids in the context of intact cells. Notably, there was also a profound rise in PtdIns3P resulting from arrested PtdIns3P consumption for PtdIns(3,5)P2 synthesis. As typical for PIKfyve inhibition and the concomitant PtdIns(3,5)P2 reduction, apilimod induced the appearance of dilated endomembrane structures in the form of large translucent cytoplasmic vacuoles. Remarkably, bafilomycin A1 (BafA1) fully reversed the aberrant cell phenotype back to normal and completely precluded the appearance of cytoplasmic vacuoles when added prior to apilimod. Inspection of the PI profiles ruled out restoration of the reduced PtdIns(3,5)P2 pool as a molecular mechanism underlying BafA1 rescue. Rather, we found that BafA1 markedly attenuated the PtdIns3P elevation under PIKfyve inhibition. This was accompanied by profoundly decreased endosomal recruitment of fusogenic EEA1. Together, our data demonstrate that apilimod inhibits not only PtdIns(3,5)P2 but also PtdIns5P synthesis and that the cytoplasmic vacuolization triggered by the inhibitor is precluded or reversed by BafA1 through a mechanism associated, in part, with reduction in both PtdIns3P levels and EEA1 membrane recruitment.
中文翻译:

Apilimod 是一种候选抗癌药物,不仅阻止 PtdIns(3,5)P2,还阻止 PIKfyve 合成 PtdIns5P,并诱导巴弗洛霉素 A1 可逆的异常内膜扩张
PIKfyve 是一种进化上保守的激酶,可合成 PtdIns5P 和 PtdIns(3,5)P 2,对哺乳动物细胞增殖和活力至关重要。因此,PIKfyve 抑制剂目前正作为抗癌药物进行临床试验。其中,apilimod 是最有前途的,但其抑制 PIKfyve 和影响内膜稳态的效力仅得到部分表征。我们在此首次证明 apilimod 与 PtdIns(3,5)P 2 的体外合成一起强力抑制 PtdIns5P 的体外合成。基于 HPLC 的细胞内磷酸肌醇 (PI) 分辨率显示,在完整细胞的背景下,apilimod 引发了两种脂质的显着减少。值得注意的是,由于 PtdIns(3,5)P 的 PtdIns3P 消耗被抑制,PtdIns3P 也出现了大幅上升2合成。作为 PIKfyve 抑制和伴随的 PtdIns(3,5)P 2减少的典型特征,apilimod 诱导扩张的内膜结构以大的半透明细胞质液泡的形式出现。值得注意的是,巴弗洛霉素 A1 (BafA1) 将异常细胞表型完全逆转回正常,并在 apilimod 之前添加时完全排除了细胞质空泡的出现。检查 PI 曲线排除了还原的 PtdIns(3,5)P 2池作为 BafA1 拯救的分子机制。相反,我们发现 BafA1 在 PIKfyve 抑制下显着减弱了 PtdIns3P 的升高。这伴随着融合EEA1的内体募集显着减少。总之,我们的数据表明,apilimod 不仅抑制 PtdIns(3,5)P 2还抑制 PtdIns5P 合成,并且 BafA1 通过与 PtdIns3P 减少相关的机制排除或逆转了抑制剂引发的细胞质空泡化水平和 EEA1 膜募集。


























 京公网安备 11010802027423号
京公网安备 11010802027423号