Joule ( IF 39.8 ) Pub Date : 2018-09-21 , DOI: 10.1016/j.joule.2018.09.002 Aliza Khurram , Mingfu He , Betar M. Gallant
|
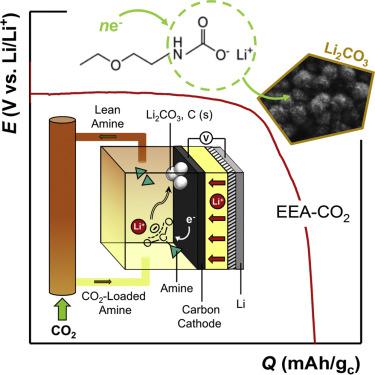
The search for viable end-uses of CO2 has motivated considerable research into CO2 utilization in energy storage devices such as alkali metal-based O2/CO2 and -CO2 batteries. However, efforts have been stymied by the low electrochemical activity of CO2 in most organic media. In this work, we report a mediated CO2 capture and conversion process, based on amine (e.g., 2-ethoxyethylamine) chemisorption, which provides a new electrolyte system for facilitating the discharge reaction in Li-CO2 batteries. Our results indicate that electrochemical reduction of CO2-loaded amines proceeds at significantly higher discharge potentials (∼2.9 V versus Li/Li+) compared with physically dissolved CO2, which is inactive in the amine's absence. The discharge reaction forms solid-phase Li2CO3 as the primary discharge product and yields high discharge capacities (>1,000 mAh/gc), highlighting the coupling of CO2 capture chemistry to nonaqueous batteries as a promising approach for the design and manipulation of CO2 conversion reactions.
中文翻译:

通过结合CO 2捕获化学来调整Li-CO 2电池中的放电反应
对CO的可行的最终用途的搜索2已促使大量的研究成CO 2的利用率在能量存储装置,例如碱金属-O 2 / CO 2和-CO 2电池。然而,在大多数有机介质中,CO 2的电化学活性低已阻碍了人们的努力。在这项工作中,我们报告了一种基于胺(例如2-乙氧基乙胺)化学吸附的介导的CO 2捕获和转化过程,该过程为促进Li-CO 2电池的放电反应提供了一种新的电解质体系。我们的结果表明,CO 2的电化学还原与物理溶解的CO 2相比,负载了胺的胺在明显更高的放电电势下(相对于Li / Li +约为2.9 V )进行放电,而在胺不存在的情况下,CO 2是无活性的。放电反应形成固相Li 2 CO 3作为主要放电产物,并产生高放电容量(> 1,000 mAh / g c),这突出表明了CO 2捕集化学物质与非水电池的耦合是设计和操作的一种有前途的方法CO 2转化反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号