当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Phosphine‐Catalyzed [4+1] Annulations of o‐Quinone Methides with MBH Carbonates
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-10-22 , DOI: 10.1002/adsc.201801152 Tao Zhou 1 , Tong Xia 1 , Zhenli Liu 1 , Lu Liu 1 , Junliang Zhang 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-10-22 , DOI: 10.1002/adsc.201801152 Tao Zhou 1 , Tong Xia 1 , Zhenli Liu 1 , Lu Liu 1 , Junliang Zhang 1
Affiliation
|
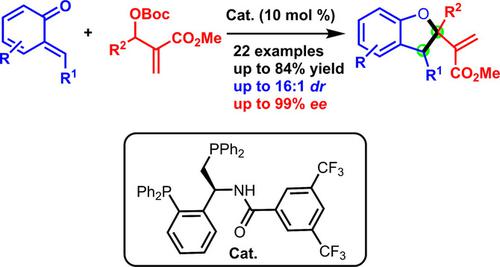
Chiral dihydrobenzofuran units are frequently present in molecules with significant biological and pharmaceutical activities. Herein, we present the first enantioselective formal [4+1] annulation of Morita‐Baylis‐Hillman carbonates with o‐quinone methides (o‐QMs) catalyzed by a newly designed chiral phosphine catalyst. Under the mild and eco‐friendly conditions, a wide range of polysubstituted dihydrobenzofurans were obtained in good yields with excellent enantioselectivities.
中文翻译:

非对称膦催化的邻苯二甲酰甲烷与MBH碳酸盐的环化[4 + 1]环
手性二氢苯并呋喃单元经常存在于具有重要生物学和药学活性的分子中。在本文中,我们介绍了新设计的手性膦催化剂催化的森田贝利斯-希尔曼碳酸盐与邻醌甲基化物(o- QMs)的首次对映选择性形式[4 + 1]环化。在温和和生态友好的条件下,以良好的收率和优异的对映选择性获得了多种多取代的二氢苯并呋喃。
更新日期:2018-10-22
中文翻译:

非对称膦催化的邻苯二甲酰甲烷与MBH碳酸盐的环化[4 + 1]环
手性二氢苯并呋喃单元经常存在于具有重要生物学和药学活性的分子中。在本文中,我们介绍了新设计的手性膦催化剂催化的森田贝利斯-希尔曼碳酸盐与邻醌甲基化物(o- QMs)的首次对映选择性形式[4 + 1]环化。在温和和生态友好的条件下,以良好的收率和优异的对映选择性获得了多种多取代的二氢苯并呋喃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号