当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computationally Assisted Mechanistic Investigation and Development of Pd-Catalyzed Asymmetric Suzuki–Miyaura and Negishi Cross-Coupling Reactions for Tetra-ortho-Substituted Biaryl Synthesis
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acscatal.8b02509 Nitinchandra D. Patel 1 , Joshua D. Sieber 1, 2 , Sergei Tcyrulnikov 3 , Bryan J. Simmons 4 , Daniel Rivalti 1, 5 , Krishnaja Duvvuri 6 , Yongda Zhang 1 , Donghong A. Gao 1 , Keith R. Fandrick 1 , Nizar Haddad 1 , Kendricks So Lao 7 , Hari P. R. Mangunuru 1, 5 , Soumik Biswas 1 , Bo Qu 1 , Nelu Grinberg 1 , Scott Pennino 8 , Heewon Lee 1 , Jinhua J. Song 1 , B. Frank Gupton 5 , Neil K. Garg 4 , Marisa C. Kozlowski 3 , Chris H. Senanayake 1, 9
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-09-20 00:00:00 , DOI: 10.1021/acscatal.8b02509 Nitinchandra D. Patel 1 , Joshua D. Sieber 1, 2 , Sergei Tcyrulnikov 3 , Bryan J. Simmons 4 , Daniel Rivalti 1, 5 , Krishnaja Duvvuri 6 , Yongda Zhang 1 , Donghong A. Gao 1 , Keith R. Fandrick 1 , Nizar Haddad 1 , Kendricks So Lao 7 , Hari P. R. Mangunuru 1, 5 , Soumik Biswas 1 , Bo Qu 1 , Nelu Grinberg 1 , Scott Pennino 8 , Heewon Lee 1 , Jinhua J. Song 1 , B. Frank Gupton 5 , Neil K. Garg 4 , Marisa C. Kozlowski 3 , Chris H. Senanayake 1, 9
Affiliation
|
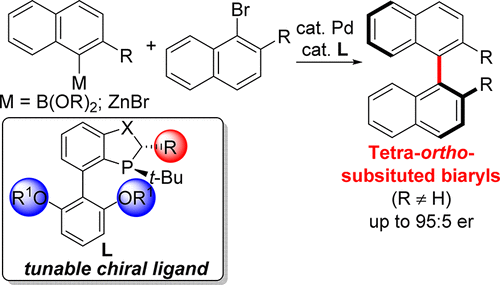
Metal-catalyzed cross-coupling reactions are extensively employed in both academia and industry for the synthesis of biaryl derivatives for applications to both medicine and material science. Application of these methods to prepare tetra-ortho-substituted biaryls leads to chiral atropisomeric products that introduce the opportunity to use catalyst control to develop asymmetric cross-coupling procedures to access these important compounds. Asymmetric Pd-catalyzed Suzuki–Miyaura and Negishi cross-coupling reactions to form tetra-ortho-substituted biaryls were studied employing a collection of P-chiral dihydrobenzooxaphosphole (BOP) and dihydrobenzoazaphosphole (BAP) ligands. Enantioselectivities of up to 95:5 and 85:15 enantiomeric ratios were identified for the Suzuki–Miyaura and Negishi cross-coupling reactions, respectively. Unique ligands for the Suzuki–Miyaura reaction vs the Negishi reaction were identified. A computational study on these Suzuki–Miyaura and Negishi cross-coupling reactions enabled an understanding in the differences between the enantiodiscriminating events between these two cross-coupling reactions. These results support that enantioselectivity in the Negishi reaction results from the reductive elimination step, whereas all steps in the Suzuki–Miyaura catalytic cycle contribute to the overall enantioselection with transmetalation and reductive elimination providing the most contribution to the observed selectivities.
中文翻译:

Pd催化的不对称Suzuki-Miyaura与Negishi交叉偶联反应的计算辅助机理研究与开发,用于四邻位取代联芳基的合成
金属催化的交叉偶联反应在学术界和工业界广泛用于合成联芳基衍生物,用于医学和材料科学。这些方法在制备四邻位取代的联芳基中的应用导致手性阻转异构产物,这为使用催化剂控制开发不对称的交叉偶联程序提供了机会,以获取这些重要的化合物。研究了不对称Pd催化的Suzuki-Miyaura和Neshishi交叉偶联反应形成四邻位取代的联芳基的过程,方法是收集P-手性二氢苯并恶唑磷(BOP)和二氢苯并氮杂磷(BAP)配体。Suzuki-Miyaura和Negishi交叉偶联反应的对映体选择性分别高达95:5和85:15。确定了铃木-宫浦反应与根岸反应的独特配体。对这些Suzuki-Miyaura和Negishi交叉偶联反应的计算研究使我们能够理解这两个交叉偶联反应之间的对映区别事件之间的差异。这些结果表明,根除反应中对映选择性是由还原消除步骤产生的,
更新日期:2018-09-20
中文翻译:

Pd催化的不对称Suzuki-Miyaura与Negishi交叉偶联反应的计算辅助机理研究与开发,用于四邻位取代联芳基的合成
金属催化的交叉偶联反应在学术界和工业界广泛用于合成联芳基衍生物,用于医学和材料科学。这些方法在制备四邻位取代的联芳基中的应用导致手性阻转异构产物,这为使用催化剂控制开发不对称的交叉偶联程序提供了机会,以获取这些重要的化合物。研究了不对称Pd催化的Suzuki-Miyaura和Neshishi交叉偶联反应形成四邻位取代的联芳基的过程,方法是收集P-手性二氢苯并恶唑磷(BOP)和二氢苯并氮杂磷(BAP)配体。Suzuki-Miyaura和Negishi交叉偶联反应的对映体选择性分别高达95:5和85:15。确定了铃木-宫浦反应与根岸反应的独特配体。对这些Suzuki-Miyaura和Negishi交叉偶联反应的计算研究使我们能够理解这两个交叉偶联反应之间的对映区别事件之间的差异。这些结果表明,根除反应中对映选择性是由还原消除步骤产生的,


























 京公网安备 11010802027423号
京公网安备 11010802027423号