当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
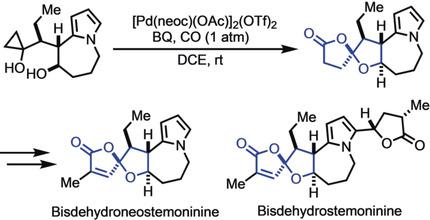
Total Syntheses of Bisdehydroneostemoninine and Bisdehydrostemoninine by Catalytic Carbonylative Spirolactonization
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-10-18 , DOI: 10.1002/anie.201809114 Kaiqing Ma 1, 2 , Xianglin Yin 1 , Mingji Dai 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-10-18 , DOI: 10.1002/anie.201809114 Kaiqing Ma 1, 2 , Xianglin Yin 1 , Mingji Dai 1
Affiliation
|
The first total syntheses of the stemona alkaloids bisdehydroneostemoninine and bisdehydrostemoninine in racemic forms have been achieved. The synthetic strategy features a novel palladium‐catalyzed carbonylative spirolactonization of a hydroxycyclopropanol to rapidly construct the oxaspirolactone moiety. It also features a Lewis acid promoted tandem Friedel–Crafts cyclization and lactonization to form the 5‐7‐5 tricyclic core of the target stemona alkaloids.
中文翻译:

催化羰基化螺内酯化全合成双脱氢百部宁和双脱氢百部宁
首次全合成百部生物碱双脱氢百部宁和外消旋形式的双脱氢百部宁。该合成策略采用新型钯催化羟基环丙醇的羰基化螺内酯化反应来快速构建氧杂螺内酯部分。它还具有路易斯酸促进串联弗里德尔-克拉夫茨环化和内酯化作用,形成目标百部生物碱的 5-7-5 三环核心。
更新日期:2018-10-18
中文翻译:

催化羰基化螺内酯化全合成双脱氢百部宁和双脱氢百部宁
首次全合成百部生物碱双脱氢百部宁和外消旋形式的双脱氢百部宁。该合成策略采用新型钯催化羟基环丙醇的羰基化螺内酯化反应来快速构建氧杂螺内酯部分。它还具有路易斯酸促进串联弗里德尔-克拉夫茨环化和内酯化作用,形成目标百部生物碱的 5-7-5 三环核心。


























 京公网安备 11010802027423号
京公网安备 11010802027423号