当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A concise synthesis of (E)-3-aryl-2,3,4,5-tetrahydro-1H-3-benzazonines by aryne induced [2,3] Stevens rearrangement of 1,2,3,4-tetrahydroisoquinolines
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-20 , DOI: 10.1039/c8ob02099j Xuan Pan 1, 2, 3, 4, 5 , Yantao Ma 1, 2, 3, 4, 5 , Zhanzhu Liu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-09-20 , DOI: 10.1039/c8ob02099j Xuan Pan 1, 2, 3, 4, 5 , Yantao Ma 1, 2, 3, 4, 5 , Zhanzhu Liu 1, 2, 3, 4, 5
Affiliation
|
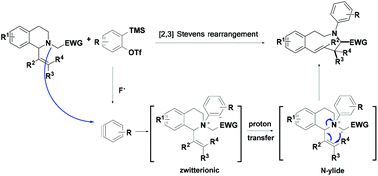
A mild and transition-metal-free approach for the synthesis of (E)-3-aryl-2,3,4,5-tetrahydro-1H-3-benzazonines via [2,3] Stevens rearrangement of 1,2,3,4-tetrahydroisoquinolines with arynes is described. This protocol provides straightforward access to (E)-3-aryl-2,3,4,5-tetrahydro-1H-3-benzazonines in moderate to good yields with excellent diastereoselectivity. A broad range of functional groups, involving nitrile, esters, ketone and aryl halide, were well tolerated in this reaction.
中文翻译:

芳烃诱导的[2,3] 1,2,3,4-四氢异喹啉的史蒂文斯重排,可合成简明的(E)-3-芳基-2,3,4,5-四氢-1 H -3-苯并zon嗪
用于合成的温和和过渡金属-自由的方法(ê)-3-芳基-2,3,4,5-四氢-1H-1 ħ -3- benzazonines通过[2,3]的1,2-史蒂文斯重排,描述了具有芳烃的3,4-四氢异喹啉。该协议提供了以中等到良好的产率和优异的非对映选择性直接获得(E)-3-芳基-2,3,4,5-四氢-1 H -3-苯并zon嗪的方法。在该反应中,对腈,酯,酮和卤代芳基等多种官能团的耐受性良好。
更新日期:2018-10-18
中文翻译:

芳烃诱导的[2,3] 1,2,3,4-四氢异喹啉的史蒂文斯重排,可合成简明的(E)-3-芳基-2,3,4,5-四氢-1 H -3-苯并zon嗪
用于合成的温和和过渡金属-自由的方法(ê)-3-芳基-2,3,4,5-四氢-1H-1 ħ -3- benzazonines通过[2,3]的1,2-史蒂文斯重排,描述了具有芳烃的3,4-四氢异喹啉。该协议提供了以中等到良好的产率和优异的非对映选择性直接获得(E)-3-芳基-2,3,4,5-四氢-1 H -3-苯并zon嗪的方法。在该反应中,对腈,酯,酮和卤代芳基等多种官能团的耐受性良好。


























 京公网安备 11010802027423号
京公网安备 11010802027423号