Chemical Physics ( IF 2.3 ) Pub Date : 2018-09-18 , DOI: 10.1016/j.chemphys.2018.09.014 Q. Sun , X.W. Su , C.B. Cheng
|
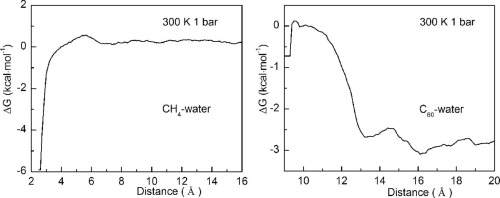
The dependence of hydrophobic interactions as a function of solute size has been investigated as an extension to our recent study detailing the physical origin of hydrophobic interactions. By determining the hydration free energy of two identical hydrophobic solutes dissolved in water, the critical radius (Rc) was calculated to be 3.25 Å, at ambient conditions. With reference to Rc, an initial process and a hydrophobic solvation process have been shown to govern the behavior of the dissolved solutes. These processes can be demonstrated by molecular dynamic simulations on C60-C60 fullerenes in water, and CH4 dimers in water. In the case of the former, by decreasing the distance between the C60 fullerene molecules, the hydrophobic interactions switch between H1w- and H2s-type hydrophobic processes. Additionally, maximizing hydrogen bonding was found to be the driving force that influenced the behavior of the hydrophobic solutes.
中文翻译:

疏水相互作用对溶质尺寸的依赖性
已经研究了疏水相互作用与溶质大小的关系,作为对我们最近研究的扩展,该研究详细描述了疏水相互作用的物理起源。通过确定两种相同的溶于水的疏水溶质的水合自由能,在环境条件下的临界半径(Rc)计算为3.25Å。关于Rc,已经显示出初始过程和疏水溶剂化过程控制着溶解的溶质的行为。这些过程可以通过对水中的C 60 -C 60富勒烯和水中的CH 4二聚体进行分子动力学模拟来证明。在前者的情况下,通过减小C 60之间的距离富勒烯分子之间,疏水相互作用在H1w型和H2s型疏水过程之间切换。此外,发现最大程度的氢键是影响疏水性溶质行为的驱动力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号